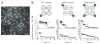Subunit counting in membrane-bound proteins - PubMed (original) (raw)
Subunit counting in membrane-bound proteins
Maximilian H Ulbrich et al. Nat Methods. 2007 Apr.
Abstract
The subunit number and stoichiometry of membrane-bound proteins are difficult to determine without disrupting their membrane environment. Here we describe a single-molecule technique for counting subunits of proteins in live cell membranes by observing bleaching steps of GFP fused to a protein of interest. After testing the method with proteins of known stoichiometry expressed in Xenopus laevis oocytes, we resolved the composition of NMDA receptors composed of NR1 and NR3 subunits.
Conflict of interest statement
Competing Interests Statement: The authors declare no competing financial interests.
Figures
Figure 1
Bleaching steps of single fluorescent protein complexes reveal the number of GFP-labeled subunits. (a) A single image of the acquired sequence shows the selected fluorescent spots in the cell membrane (blue circles). Scale bar, 2 μm. (b) The different labeling schemes include 1, 2 or 4 subunits of a membrane protein complex fused to GFP. (c) Time courses of fluorescence emission for the labeling schemes in b (two examples each from spots bleaching with the expected number of bleaching steps). Green arrows indicate the bleaching steps. The _y_-axis is scaled in photons per second for comparison purposes (for calibration see Supplementary Methods).
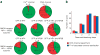
Figure 2
Distribution of bleaching steps for channels with different numbers of labeled subunits. (a) Numbers A × B in the sectors indicate that A spots with B bleaching steps were observed. Green sector, number of bleaching steps expected from biochemical and functional studies. Red sectors, number of bleaching steps different from what is expected. (b) The observed numbers of spots having 1, 2, 3 and 4 bleaching steps in the experiment with the tetrameric CNG channel (red) match closely a calculated binomial distribution (blue), assuming a probability of 77.5% that the GFP is fluorescent.
Similar articles
- Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function.
Madry C, Mesic I, Bartholomäus I, Nicke A, Betz H, Laube B. Madry C, et al. Biochem Biophys Res Commun. 2007 Mar 2;354(1):102-8. doi: 10.1016/j.bbrc.2006.12.153. Epub 2006 Dec 28. Biochem Biophys Res Commun. 2007. PMID: 17214961 - Rules of engagement for NMDA receptor subunits.
Ulbrich MH, Isacoff EY. Ulbrich MH, et al. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14163-8. doi: 10.1073/pnas.0802075105. Epub 2008 Sep 8. Proc Natl Acad Sci U S A. 2008. PMID: 18779583 Free PMC article. - The molecular diversity of glutamate receptors.
Nakanishi S. Nakanishi S. Prog Clin Biol Res. 1994;390:85-98. Prog Clin Biol Res. 1994. PMID: 7724653 Review. No abstract available. - Ion currents of Xenopus laevis oocytes: state of the art.
Weber W. Weber W. Biochim Biophys Acta. 1999 Oct 15;1421(2):213-33. doi: 10.1016/s0005-2736(99)00135-2. Biochim Biophys Acta. 1999. PMID: 10518693 Review. No abstract available.
Cited by
- Breakage of the oligomeric CaMKII hub by the regulatory segment of the kinase.
Karandur D, Bhattacharyya M, Xia Z, Lee YK, Muratcioglu S, McAffee D, McSpadden ED, Qiu B, Groves JT, Williams ER, Kuriyan J. Karandur D, et al. Elife. 2020 Sep 9;9:e57784. doi: 10.7554/eLife.57784. Elife. 2020. PMID: 32902386 Free PMC article. - Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins.
Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Siebert AP, et al. J Biol Chem. 2013 Mar 1;288(9):6140-53. doi: 10.1074/jbc.M112.409789. Epub 2013 Jan 8. J Biol Chem. 2013. PMID: 23300080 Free PMC article. - Identification of an inhibitory mechanism of luteolin on the insulin-like growth factor-1 ligand-receptor interaction.
Yang Y, Shen J, Yu X, Qin G, Zhang M, Shen H, Mao Z, Ferrari M. Yang Y, et al. Chembiochem. 2013 May 27;14(8):929-33. doi: 10.1002/cbic.201300082. Epub 2013 Apr 29. Chembiochem. 2013. PMID: 23630137 Free PMC article. - The conserved tetrameric subunit stoichiometry of Slc26 proteins.
Hallworth R, Stark K, Zholudeva L, Currall BB, Nichols MG. Hallworth R, et al. Microsc Microanal. 2013 Aug;19(4):799-807. doi: 10.1017/S1431927613000457. Epub 2013 May 3. Microsc Microanal. 2013. PMID: 23642772 Free PMC article. - From Monomers to Hexamers: A Theoretical Probability of the Neighbor Density Approach to Dissect Protein Oligomerization in Cells.
Chen H, Chen TY. Chen H, et al. Anal Chem. 2024 Jan 16;96(2):895-903. doi: 10.1021/acs.analchem.3c04728. Epub 2023 Dec 29. Anal Chem. 2024. PMID: 38156958 Free PMC article.
References
- MacKinnon R, Aldrich RW, Lee AW. Science. 1993;262:757–759. - PubMed
- Liman ER, Tytgat J, Hess P. Neuron. 1992;9:861–871. - PubMed
- Doyle DA, et al. Science. 1998;280:69–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources