Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia - PubMed (original) (raw)
Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia
Kalindi Parmar et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The interaction of stem cells with their bone marrow microenvironment is a critical process in maintaining normal hematopoiesis. We applied an approach to resolve the spatial organization that underlies these interactions by evaluating the distribution of hematopoietic cell subsets along an in vivo Hoechst 33342 (Ho) dye perfusion gradient. Cells isolated from different bone marrow regions according to Ho fluorescence intensity contained the highest concentration of hematopoietic stem cell (HSC) activity in the lowest end of the Ho gradient (i.e., in the regions reflecting diminished perfusion). Consistent with the ability of Ho perfusion to simulate the level of oxygenation, bone marrow fractions separately enriched for HSCs were found to be the most positive for the binding of the hypoxic marker pimonidazole. Moreover, the in vivo administration of the hypoxic cytotoxic agent tirapazamine exhibited selective toxicity to the primitive stem cell subset. These data collectively indicate that HSCs and the supporting cells of the stem cell niche are predominantly located at the lowest end of an oxygen gradient in the bone marrow with the implication that regionally defined hypoxia plays a fundamental role in regulating stem cell function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
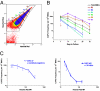
Separation of different bone marrow cell (BMC) fractions according to a Ho dye diffusion gradient. (A) Blue versus red fluorescence intensity of BMCs after i.v. infusion of Ho dye (0.8 mg per mouse at 5 and 10 min before killing) with R2-R7 gates representing sorted cell fractions of diminishing fluorescence. (B) CAFC frequencies versus time in culture for the different cell fractions. (C) Early- and late-forming CAFC frequencies as a function of Ho red mean fluorescence intensity (MFI) determined from analysis of postsorted cells. Downward arrows indicate that the CAFC frequencies were below the limit of detection.
Fig. 2.
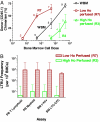
Long-term repopulation for donor cells (CD45.1) sorted from high (R3) and low (R7) Ho-perfused bone marrow. Sorted bone marrow cells from donor (CD45.1) mice were transplanted into 10 Gy irradiated recipients (CD45.2) along with 200,000 recipient-type, non-SP cells to provide for short-term repopulation. Donor cell chimerism in the recipient mice was analyzed at 18 weeks and 12–14 months posttransplant. (A) Myeloid engraftment in the blood after 18 weeks posttransplant is shown. The number of mice for each cell dose group is indicated. The frequencies of repopulating cells (LTRUs) capable of producing >50% myeloid engraftment with 95% confidence limits (L-Calc program; Stem Cell Technologies) were 67 (27–166), 4.5 (–10), and 1.1 (0.34–3.35) per 106 sorted cells from R7, whole Ho gradient (WBM), and R3 regions, respectively. (B) High (R3) versus low (R7) Ho dye perfused bone marrow populations for frequency of in vivo 12–14 month posttransplant LTRUs. Frequencies of donor cell chimerism in peripheral blood (PB) T cells, PB myeloid cells, bone marrow (BM) c-kit+ cells, and BM LTC-CFC are shown. Error bars represent 95% confidence limits.
Fig. 3.
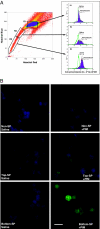
PIM adducts in vivo in bone marrow SP cells. Mice were injected with saline or 120 mg/kg PIM (n = 5), and after 3 h, bone marrow samples were collected and subjected to Ho staining in vitro to isolate SP cells. Representative PIM staining of SP and non-SP cells in the bone marrow is shown. Three different regions of bone marrow cells based on Ho dye efflux, non-SP (R1), top SP (R2), and bottom SP (R3) were sorted. The sorted cells were analyzed for PIM binding by using flow cytometry (A) as well as immunofluorescence microscopy (B). (A) For flow cytometric analysis of PIM binding, the sorted cells were fixed and intracellularly stained by using anti-PIM primary antibody and a goat anti-mouse IgG F(ab′)2 Alexa Fluor 488 secondary antibody. Similar positive staining of SP cells were obtained in two other separate experiments, including cells stained by using the FITC-conjugated anti-PIM antibody. (B) For visualization of PIM binding by microscopy, cytospin slides of non-SP, top SP, and bottom “tip” SP were immunostained for the adduct. Representative images stained for PIM (green) and nucleus (DAPI, blue) are shown. (Scale bar: 10 μM.)
Fig. 4.
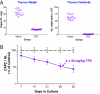
The hypoxic cytotoxin TPZ is highly toxic to thymus and selectively depletes late-forming CAFCs in the bone marrow. TPZ was administered i.p. to C57BL/6J recipients in four daily doses of 30 mg/kg. On the day after the last dose, thymus and bone marrow were harvested and compared with cells obtained from saline-injected control mice. (A) Thymus weight (Left) and cell yield (Right) (trypan blue excluding) were estimated from three separate experiments in mice treated with 4 × 30 mg/kg over 4 days showing marked toxicity of TPZ. (B) Bone marrow cells were pooled from four mice and plated for estimate of CAFC content per hind limb (HL). Error bars represent 95% confidence intervals.
Similar articles
- Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches.
Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Lévesque JP. Winkler IG, et al. Blood. 2010 Jul 22;116(3):375-85. doi: 10.1182/blood-2009-07-233437. Epub 2010 Apr 14. Blood. 2010. PMID: 20393133 - Hypoxic niche-mediated regeneration of hematopoiesis in the engraftment window is dominantly affected by oxygen tension in the milieu.
Moirangthem RD, Singh S, Adsul A, Jalnapurkar S, Limaye L, Kale VP. Moirangthem RD, et al. Stem Cells Dev. 2015 Oct 15;24(20):2423-36. doi: 10.1089/scd.2015.0112. Epub 2015 Jul 28. Stem Cells Dev. 2015. PMID: 26107807 Free PMC article. - Assessing Cellular Hypoxic Status In Situ Within the Bone Marrow Microenvironment.
Suessbier U, Nombela-Arrieta C. Suessbier U, et al. Methods Mol Biol. 2019;2017:123-134. doi: 10.1007/978-1-4939-9574-5_10. Methods Mol Biol. 2019. PMID: 31197773 - The hematopoietic stem cell niche: low in oxygen but a nice place to be.
Eliasson P, Jönsson JI. Eliasson P, et al. J Cell Physiol. 2010 Jan;222(1):17-22. doi: 10.1002/jcp.21908. J Cell Physiol. 2010. PMID: 19725055 Review. - Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche.
Heazlewood SY, Oteiza A, Cao H, Nilsson SK. Heazlewood SY, et al. Ann N Y Acad Sci. 2014 Mar;1310:119-28. doi: 10.1111/nyas.12329. Epub 2014 Jan 15. Ann N Y Acad Sci. 2014. PMID: 24428368 Review.
Cited by
- Metabolic plasticity and hematopoietic stem cell biology.
Hsu P, Qu CK. Hsu P, et al. Curr Opin Hematol. 2013 Jul;20(4):289-94. doi: 10.1097/MOH.0b013e328360ab4d. Curr Opin Hematol. 2013. PMID: 23615055 Free PMC article. Review. - Detection of Mycobacterium tuberculosis DNA in CD34 + peripheral blood mononuclear cells of Ugandan adults with latent infection: a cross-sectional and nested prospective study.
Mayito J, Andia Biraro I, T Reece S, R Martineau A, P Kateete D. Mayito J, et al. AAS Open Res. 2020 Jul 29;3:34. doi: 10.12688/aasopenres.13108.1. eCollection 2020. AAS Open Res. 2020. PMID: 32832853 Free PMC article. - Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance.
Tuy K, Rickenbacker L, Hjelmeland AB. Tuy K, et al. Redox Biol. 2021 Aug;44:101953. doi: 10.1016/j.redox.2021.101953. Epub 2021 Mar 27. Redox Biol. 2021. PMID: 34052208 Free PMC article. Review. - Noninvasive low-level laser therapy for thrombocytopenia.
Zhang Q, Dong T, Li P, Wu MX. Zhang Q, et al. Sci Transl Med. 2016 Jul 27;8(349):349ra101. doi: 10.1126/scitranslmed.aaf4964. Sci Transl Med. 2016. PMID: 27464749 Free PMC article. - Differential hypoxic regulation of the microRNA-146a/CXCR4 pathway in normal and leukemic monocytic cells: impact on response to chemotherapy.
Spinello I, Quaranta MT, Paolillo R, Pelosi E, Cerio AM, Saulle E, Lo Coco F, Testa U, Labbaye C. Spinello I, et al. Haematologica. 2015 Sep;100(9):1160-71. doi: 10.3324/haematol.2014.120295. Epub 2015 Jun 4. Haematologica. 2015. PMID: 26045293 Free PMC article. Clinical Trial.
References
- Schofield R. Blood Cells. 1978;4:7–25. - PubMed
- Weissman IL, Anderson DJ, Gage F. Annu Rev Cell Dev Biol. 2001;17:387–403. - PubMed
- Lord BI. Int J Cell Cloning. 1990;8:317–331. - PubMed
- Nilsson SK, Johnston HM, Coverdale JA. Blood. 2001;97:2293–2299. - PubMed
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19AI067751/AI/NIAID NIH HHS/United States
- R01HL073714/HL/NHLBI NIH HHS/United States
- R01 HL073714/HL/NHLBI NIH HHS/United States
- U19 AI067751/AI/NIAID NIH HHS/United States
- R01CA10941/CA/NCI NIH HHS/United States
- R01 CA010941/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous