Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation - PubMed (original) (raw)
Review
Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation
Jaswinder K Sethi et al. J Lipid Res. 2007 Jun.
Abstract
This review focuses on adipose tissue biology and introduces the concept of adipose tissue plasticity and expandability as key determinants of obesity-associated metabolic dysregulation. This concept is fundamental to our understanding of adipose tissue as a dynamic organ at the center of nutritional adaptation. Here, we summarize the current knowledge of the mechanisms by which adipose tissue can affect peripheral energy homeostasis, particularly in the context of overnutrition. Two mechanisms emerge that provide a molecular understanding for obesity-associated insulin resistance. These are a) the dysregulation of adipose tissue expandability and b) the abnormal production of adipokines. This knowledge has the potential to pave the way for novel therapeutic concepts and strategies for managing and/or correcting complications associated with obesity and the metabolic syndrome.
Figures
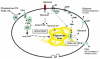
Fig. 1
Lipid metabolism in adipocytes. Adipocytes are equipped with the biochemical machinery to function effectively as the body’s fuel store. To do this, it must mediate lipogenesis [conversion of FFA to triglycerides (TG) for storage] and lipolysis (breakdown of triglycerides to FFA and glycerol). It is also sensitive to changing nutritional cues. For example, it is insulin-sensitive [insulin stimulates glucose uptake and lipogenesis and inhibits lipolysis] and subject to adrenergic regulation [stimulates lipolysis and adaptive thermogenesis (brown adipose tissue)]. AC, adenylate cyclase; ACS, acyl-CoA synthase; AKT, AKR mouse thymoma viral proto-oncogene; AR, adrenergic receptor; HSL, hormone sensitive lipase; IR, insulin receptor; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A.
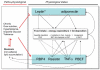
Fig. 2
The transcriptional control of adipogenesis involves the sequential activation of a transcription factor cascade. This begins with the transient expression of C/EBPβ and C/EBPδ, followed by C/EBPα, which in turn activates peroxisome proliferator-activated receptor γ 2 (PPARγ2). The latter pair is primarily responsible for switching on the broad program of adipogenesis. In addition, PPARγ exerts positive feedback on C/EBPα and acts synergistically to maintain the differentiated state. Sterol-regulatory element binding protein 1c (SREBP1c) is regulated by insulin and lipids and can activate PPARγ by inducing its expression as well as by promoting the production of an endogenous PPAR ligand. SREBP1c also activates the expression of many genes of the lipogenic program. Together, these factors contribute to the expression of genes that characterize the terminally differentiated phenotype. GATA, GATA binding protein; IBMX, 3-Isobutyl-1-methylxanthine; PEPCK, phosphoenolpyruvate carboxykinase; TZD, thiazolidinedione.
Fig. 3
Impact of adipokines on thermodynamic balance during physiological states and states of overnutrition. Potential actions in normal physiology are indicated for each adipokine. However, in pathophysiological states associated with overnutrition, these may be separated into two functional groups: adipokines whose levels or effective activity (*) are decreased (upper panel) and adipokines whose levels and/or activity are increased (lower panel). Solid lines indicate evidence for direct action. Dashed lines are indicative of putative or potential for indirect mechanisms involving other adipokines or extracellular mediators. CNS, central nervous system; TNF, tumor necrosis factor; WAT, white adipose tissue. PBEF, pre-B-cell colony enhancing factor; RBP-4, retinol binding protein-4.
Similar articles
- Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome.
Carobbio S, Pellegrinelli V, Vidal-Puig A. Carobbio S, et al. Adv Exp Med Biol. 2017;960:161-196. doi: 10.1007/978-3-319-48382-5_7. Adv Exp Med Biol. 2017. PMID: 28585199 Review. - Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance.
Murdolo G, Piroddi M, Luchetti F, Tortoioli C, Canonico B, Zerbinati C, Galli F, Iuliano L. Murdolo G, et al. Biochimie. 2013 Mar;95(3):585-94. doi: 10.1016/j.biochi.2012.12.014. Epub 2012 Dec 26. Biochimie. 2013. PMID: 23274128 Review. - Adipose Tissue Dysfunction Determines Lipotoxicity and Triggers the Metabolic Syndrome: Current Challenges and Clinical Perspectives.
Carobbio S, Pellegrinelli V, Vidal-Puig A. Carobbio S, et al. Adv Exp Med Biol. 2024;1460:231-272. doi: 10.1007/978-3-031-63657-8_8. Adv Exp Med Biol. 2024. PMID: 39287854 Review. - Adipose tissue dysfunction in obesity.
Blüher M. Blüher M. Exp Clin Endocrinol Diabetes. 2009 Jun;117(6):241-50. doi: 10.1055/s-0029-1192044. Epub 2009 Apr 8. Exp Clin Endocrinol Diabetes. 2009. PMID: 19358089 Review. - Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity.
Yu BL, Zhao SP, Hu JR. Yu BL, et al. Obes Rev. 2010 Aug;11(8):560-7. doi: 10.1111/j.1467-789X.2009.00699.x. Epub 2009 Dec 21. Obes Rev. 2010. PMID: 20025694 Review.
Cited by
- A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys.
Pugh TD, Conklin MW, Evans TD, Polewski MA, Barbian HJ, Pass R, Anderson BD, Colman RJ, Eliceiri KW, Keely PJ, Weindruch R, Beasley TM, Anderson RM. Pugh TD, et al. Aging Cell. 2013 Aug;12(4):672-81. doi: 10.1111/acel.12091. Epub 2013 May 27. Aging Cell. 2013. PMID: 23607901 Free PMC article. - Chronic inflammation in obesity and the metabolic syndrome.
Monteiro R, Azevedo I. Monteiro R, et al. Mediators Inflamm. 2010;2010:289645. doi: 10.1155/2010/289645. Epub 2010 Jul 14. Mediators Inflamm. 2010. PMID: 20706689 Free PMC article. Review. - Insulin prevents fatty acid induced increase of adipocyte size.
Berger E, Géloën A. Berger E, et al. Adipocyte. 2022 Dec;11(1):510-528. doi: 10.1080/21623945.2022.2107784. Adipocyte. 2022. PMID: 35946137 Free PMC article. - TWEAK: A New Player in Obesity and Diabetes.
Vendrell J, Chacón MR. Vendrell J, et al. Front Immunol. 2013 Dec 30;4:488. doi: 10.3389/fimmu.2013.00488. Front Immunol. 2013. PMID: 24416031 Free PMC article. Review. - Immunometabolism in obese asthmatics: are we there yet?
Periyalil HA, Gibson PG, Wood LG. Periyalil HA, et al. Nutrients. 2013 Sep 10;5(9):3506-30. doi: 10.3390/nu5093506. Nutrients. 2013. PMID: 24025484 Free PMC article. Review.
References
- Casteilla L, Penicaud L, Cousin B, Calise D. Choosing an adipose tissue depot for sampling. Factors in selection and depot specificity. Methods Mol. Biol. 2001;155:1–19. - PubMed
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources