Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis - PubMed (original) (raw)
Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis
Bodil E Jönsson et al. J Clin Microbiol. 2007 May.
Abstract
Mycobacterium abscessus has been isolated increasingly often from the respiratory tracts of cystic fibrosis (CF) patients. It is not known whether these organisms are transmitted from person to person or acquired from environmental sources. Here, colony morphology and pulsed-field gel electrophoresis (PFGE) pattern were examined for 71 isolates of M. abscessus derived from 14 CF patients, three non-CF patients with chronic respiratory M. abscessus infection or colonization, one patient with mastoiditis, and four patients with infected wounds, as well as for six isolates identified as environmental contaminants in various clinical specimens. Contaminants and wound isolates mainly exhibited smooth colony morphology, while a rough colony phenotype was significantly associated with chronic airway colonization (P=0.014). Rough strains may exhibit increased airway-colonizing capacity, the cause of which remains to be determined. Examination by PFGE of consecutive isolates from the same patient showed that they all represented a single strain, even in cases where both smooth and rough isolates were present. When PFGE patterns were compared, it was shown that 24 patients had unique strains, while four patients harbored strains indistinguishable by PFGE. Two of these were siblings with CF. The other two patients, one of whom had CF, had not had contact with each other or with the siblings. Our results show that most patients colonized by M. abscessus in the airways have unique strains, indicating that these strains derive from the environment and that patient-to-patient transmission rarely occurs.
Figures
FIG. 1.
Colony morphology of M. abscessus after 5 days of incubation on horse blood agar at 37°C. The same magnification was used for both images. (Left) Type strain CCUG 20993 with smooth colonies. (Right) Strain with rough colonies from a CF patient. Images were obtained with a Nikon Coolpix 4300 and Adobe Photoshop Elements 2.0.
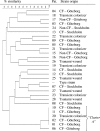
FIG. 2.
PFGE analysis of AseI-digested DNA from 29 M. abscessus isolates. Relationships among isolates from 28 Swedish patients and type strain ATCC 19977 are shown in a similarity dendrogram constructed after calculation of the similarity matrix with the Jaccard algorithm (optimization, 1.20%; position tolerance, 1.2% to 1.2%; (minimum height > 0.0%; minimum > 0.0%) and clustering according to UPGMA. CF-Göteborg, isolates obtained from CF patients in the Västra Götaland region; CF-Stockholm, isolates obtained from CF patients in the Stockholm/Uppsala area; non-CF-Göteborg, isolates from other patients with lung infection/colonization from the Västra Götaland region; non-CF-Stockholm, isolates from a patient with mastoiditis in the Stockholm area; tsunami wound, isolate from Swedish tsunami victim (strain obtained in Thailand); transient colonizer, isolate found on a single occasion in a clinical specimen followed by a negative sample and/or lack of associated clinical conditions, suggesting that the isolate was an environmental contaminant.
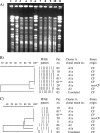
FIG. 3.
PFGE analyses of cluster A after AseI and XbaI digestion of DNA. (A) AseI PFGE gel corresponding to the dendrogram in panel B. Lanes 1, 6, and 11, reference strain S. aureus NCTC 8325 (not shown in dendrogram). Lane 2, patient 3, isolate 05*1230; lane 3, patient 6, isolate 05*2299; lane 4, patient 10, isolate 05*2316; lane 5, patient 1, isolate 01*5153; lane 7, patient 1, isolate 03*4569; lane 8, patient 2, isolate 04*2226; lane 9, patient 20, isolate 02*1284; lane 10, patient 11, isolate 01* 1526. (B and C) AseI (B) and XbaI (C) PFGE patterns presented as similarity dendrograms and schematic views of bands analyzed. *, isolate 01*5153; **, isolate 03*4569.
Similar articles
- Poor clinical outcomes associated with a multi-drug resistant clonal strain of Pseudomonas aeruginosa in the Tasmanian cystic fibrosis population.
Bradbury R, Champion A, Reid DW. Bradbury R, et al. Respirology. 2008 Nov;13(6):886-92. doi: 10.1111/j.1440-1843.2008.01383.x. Respirology. 2008. PMID: 18811887 - Enterobacterial repetitive intergenic consensus PCR is a useful tool for typing Mycobacterium chelonae and Mycobacterium abscessus isolates.
Sampaio JL, Viana-Niero C, de Freitas D, Höfling-Lima AL, Leão SC. Sampaio JL, et al. Diagn Microbiol Infect Dis. 2006 Jun;55(2):107-18. doi: 10.1016/j.diagmicrobio.2006.01.006. Epub 2006 Mar 9. Diagn Microbiol Infect Dis. 2006. PMID: 16529900 - Extrapulmonary infections caused by a dominant strain of Mycobacterium massiliense (Mycobacterium abscessus subspecies bolletii).
Cheng A, Liu YC, Chen ML, Hung CC, Tsai YT, Sheng WH, Liao CH, Hsueh PR, Chen YC, Chang SC. Cheng A, et al. Clin Microbiol Infect. 2013 Oct;19(10):E473-82. doi: 10.1111/1469-0691.12261. Epub 2013 May 30. Clin Microbiol Infect. 2013. PMID: 23718188 - Mycobacterium abscessus: an emerging rapid-growing potential pathogen.
Petrini B. Petrini B. APMIS. 2006 May;114(5):319-28. doi: 10.1111/j.1600-0463.2006.apm_390.x. APMIS. 2006. PMID: 16725007 Review. - Mycobacterium abscessus infection in cystic fibrosis: molecular typing and clinical outcomes.
Harris KA, Kenna DTD. Harris KA, et al. J Med Microbiol. 2014 Oct;63(Pt 10):1241-1246. doi: 10.1099/jmm.0.077164-0. Epub 2014 Aug 8. J Med Microbiol. 2014. PMID: 25106861 Review.
Cited by
- Dissecting erm(41)-Mediated Macrolide-Inducible Resistance in Mycobacterium abscessus.
Richard M, Gutiérrez AV, Kremer L. Richard M, et al. Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01879-19. doi: 10.1128/AAC.01879-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31791943 Free PMC article. - Mycobacterium abscessus DosRS two-component system controls a species-specific regulon required for adaptation to hypoxia.
Simcox BS, Tomlinson BR, Shaw LN, Rohde KH. Simcox BS, et al. Front Cell Infect Microbiol. 2023 Mar 9;13:1144210. doi: 10.3389/fcimb.2023.1144210. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36968107 Free PMC article. - Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin.
Palucci I, Salustri A, De Maio F, Pereyra Boza MDC, Paglione F, Sali M, Occhigrossi L, D'Eletto M, Rossin F, Goletti D, Sanguinetti M, Piacentini M, Delogu G. Palucci I, et al. Int J Mol Sci. 2023 Jan 7;24(2):1203. doi: 10.3390/ijms24021203. Int J Mol Sci. 2023. PMID: 36674717 Free PMC article. - Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential.
Choo SW, Wee WY, Ngeow YF, Mitchell W, Tan JL, Wong GJ, Zhao Y, Xiao J. Choo SW, et al. Sci Rep. 2014 Feb 11;4:4061. doi: 10.1038/srep04061. Sci Rep. 2014. PMID: 24515248 Free PMC article. - Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis.
Qvist T, Pressler T, Høiby N, Katzenstein TL. Qvist T, et al. Respir Res. 2014 Apr 11;15(1):41. doi: 10.1186/1465-9921-15-41. Respir Res. 2014. PMID: 24725650 Free PMC article.
References
- Butler, W., M. Floyd, V. Silcow, G. Cage, E. Desmond, P. Duffey, L. Guthertz, W. Gross, K. C. Jost, Jr., L. Ramos, L. Thibert, and N. Warren. 1996. Standardized method for HPLC identification of mycobacteria. Centers for Disease Control and Prevention, Atlanta, GA. http://cdc.gov/ncidod/publications/hplc.pdf.
- Chang, L., K. Hohneker, M. Knowles, P. Gilligan, and N. Peader. 2005. Mycobacterium abscessus infection in cystic fibrosis. Pediatr. Pulmonol. Suppl. 28:291-292.
- Daley, C. L., and D. E. Griffith. 2002. Pulmonary disease caused by rapidly growing mycobacteria. Clin. Chest Med. 23:623-632. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical