Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination - PubMed (original) (raw)
Comparative Study
. 2007 Jun;81(11):5819-28.
doi: 10.1128/JVI.00024-07. Epub 2007 Mar 21.
Affiliations
- PMID: 17376899
- PMCID: PMC1900286
- DOI: 10.1128/JVI.00024-07
Comparative Study
Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination
Vijayakumar Velu et al. J Virol. 2007 Jun.
Abstract
Here, we study the temporal expression of the inhibitory receptor programmed death 1 (PD-1) on simian immunodeficiency virus (SIV) Gag-specific T cells following pathogenic SIV infection or following vaccination with a DNA/modified vaccinia virus Ankara (DNA/MVA) vaccine and simian/human immunodeficiency virus (SHIV) challenge in macaques. Following infection, the majority (>95%) of Gag-specific CD8 T cells expressed PD-1, and the level of PD-1 expression per cell increased over time. The level of PD-1 expression in lymph nodes and rectal mucosal tissue, the major sites of virus replication, was higher compared to blood. In vitro blockade of PD-1 resulted in enhanced proliferation of SIV-specific CD8 as well as CD4 T cells. In contrast, following vaccination, the majority of peak effector Gag-specific CD8 T cells expressed low levels of PD-1, and these levels decreased further as the cells differentiated into memory cells. In addition, following SHIV challenge of these vaccinated macaques, the level of PD-1 expression on Gag-specific CD8 T cells correlated positively with plasma viremia. These results demonstrate that SIV-specific CD8 T cells express PD-1 after exposure to antigen but downregulate expression under conditions of antigen clearance and enhance expression under conditions of antigen persistence. They also demonstrate that the level of PD-1 expression per cell rather than the presence or absence of expression plays an important role in regulating CD8 T-cell dysfunction in pathogenic SIV infection. In addition, they demonstrate that similar to HIV infection, the PD-1:PD-1 ligand inhibitory pathway is operational in pathogenic SIV infection, and the macaque/SIV model would be ideal to test the safety and therapeutic benefit of blocking this pathway in vivo.
Figures
FIG. 1.
PD-1 expression on total and SIV Gag-specific CD8 T cells following pathogenic SIV or SHIV infection. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from a SIV251-infected macaque. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells (CD3+, CD8+) and tetramer-positive cells were analyzed for expression of PD-1. The open and filled histograms represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. The numbers on the FACS plots represent the frequency of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of frequency of PD-1-positive cells and the MFI of PD-1 on total CD8 T cells at various times following SIV251 infection. (C) Summary of frequency of PD-1-positive cells and the MFI of PD-1 on tetramer-positive cells at various times following SIV251 infection. The data represent the mean values for four macaques. Error bars represent standard deviations. (D) Summary of the frequency of PD-1-positive cells as a percentage of tetramer-positive CD8 T cells at 12 weeks following infection. (E) Fold increase in the MFI of PD-1 on Gag-CM9 tetramer-positive cells over the MFI for total CD8 T cells in respective macaques at 12 weeks after infection. Each symbol represents an individual macaque.
FIG. 2.
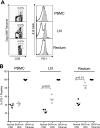
PD-1 expression on total and SIV Gag-specific CD8 T cells in lymph node and rectal mucosal tissue. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total (open histograms) and SIV Gag CM9 tetramer-specific (filled histograms) CD8 T cells in blood, lymph node, and rectal mucosal tissue from a SHIV-infected macaque. Cells were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The numbers on the FACS plots represent the frequencies of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of PD-1 expression on total and tetramer-positive CD8 T cells in blood, peripheral lymph node, and rectal mucosal tissue from normal and SHIV-infected macaques. For SHIV-infected macaques, analysis was performed at 12 weeks after infection. Each symbol represents an individual macaque.
FIG. 3.
Effect of in vitro blockade of PD-1 on SIV-specific CD8 T cells in SIV- or SHIV-infected macaques. (A) Fluorescence-activated cell sorter plots representing the frequency of Gag-CM9 tetramer-positive cells following stimulation in vitro. PBMC were prestained with CFSE and stimulated for 6 days with P11C peptide in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) in the presence and absence of blocking antibody served as negative controls. At the end of 6 days, cells were stained on the surface for CD3, CD8, and Gag-CM9 tetramer, acquired on a FACSCalibur, and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). Cells were gated on CD3 and analyzed for expression of CD8 and tetramer binding. The numbers on the plots represent the frequency of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of proliferation data for SIV- or SHIV-infected macaques. Fold increase (frequency of tetramer-positive cells in stimulated cultures over unstimulated cultures) in the frequency of tetramer-positive cells is plotted for each macaque. Each symbol represents an individual macaque. (C) Granzyme B and perforin expression on tetramer-positive cells following in vitro stimulation in the presence and absence of blocking antibody. PBMC were stimulated for 6 days with P11C peptide in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) served as negative controls. At the end of 6 days cells, were stained on the surface for CD3, CD8, and Gag-CM9 tetramer. Cells were then fixed, permeabilized, and stained for intracellular perforin and granzyme B and acquired on an LSRII apparatus. Cells were gated on CD3, CD8, and tetramer and analyzed for expression of granzyme B or perforin. The gray filled histograms and black open histograms represent expression on P11C-stimulated cells in the absence and presence of anti-PD-1 blocking antibody, respectively. The gray open histograms represent expression on total CD8 T cells in unstimulated cultures. (D) Summary of granzyme B and perforin data for three SHIV-infected macaques.
FIG. 4.
PD-1 expression on total and SIV Gag-specific CD8 T cells following vaccination with a DNA/MVA SIV vaccine. (A) Fluorescence-activated cell sorter plots representing the expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from a macaque vaccinated with DNA/MVA vaccine. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The gray filled histograms and black open histograms represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. (B) Summary of PD-1 expression on tetramer-positive CD8 T cells at the peak (1 week) and memory (6 months) phases following the MVA boost. (C) Summary of MFI of PD-1 on tetramer-positive CD8 T cells at the peak (1 week) and memory (6 months) phases following the MVA boost. (D) Effect of in vitro blockade of PD-1 on the proliferative capacity of SIV Gag CM9 tetramer-specific memory cells. Cells were stimulated, stained, and analyzed as described for Fig. 3. Fold increase (frequency of tetramer-positive cells in stimulated cultures over unstimulated cultures) in the frequency of tetramer-positive cells is plotted for each macaque. Each symbol represents an individual macaque.
FIG. 5.
PD-1 expression following SHIV challenge in unvaccinated and DNA/MVA-vaccinated macaques. (A) Viral RNA levels in plasma following SHIV challenge. (B) Fluorescence-activated cell sorter plots representing expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from unvaccinated (control) and DNA/MVA-vaccinated macaques at 2 and 12 weeks after SHIV 89.6P challenge. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The contour plots and black dots represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. The boxes on the plots represent PD-1lo (left) and PD-1hi (right) cells. The numbers on the plots represent the frequency of PD-1hi cells as a percentage of total tetramer-positive cells. (C) Summary of the frequency of PD-1-positive cells as a percentage of tetramer-positive CD8 T cells at 12 weeks following SIV or SHIV infection in unvaccinated SIV-infected (control SIV), unvaccinated SHIV-infected (control SHIV), and DNA/MVA-vaccinated SHIV-infected (vaccinated SHIV) macaques. (D) Summary of frequency of PD-1hi cells on tetramer-positive CD8 T cells at 12 weeks after SIV or SHIV infection. (E) Correlation between the frequency of PD-1hi cells and plasma viral load at 12 weeks after infection in unvaccinated SIV-infected, unvaccinated SHIV-infected, and DNA/MVA-vaccinated SHIV-infected macaques. The MFI of PD-1 on tetramer-positive cells could not be used for comparisons between groups because these analyses were performed using different batches of antibody that resulted in differences in staining intensity. Each symbol represents an individual macaque.
FIG. 6.
Effect of in vitro blockade of PD-1 on SIV-specific CD4 T cells in SIV-infected macaques. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total CD4 T cells in blood from a SIV-infected macaque. PBMC were stained on the surface with antibodies to human CD3, CD4, CD8, and PD-1. CD3+, CD4+, and CD8− cells were analyzed for expression of PD-1. (B) FACS plots representing the frequency of Gag-specific CD4 T cells following stimulation in vitro. PBMC were prestained with CFSE and stimulated for 6 days with Gag peptide pool in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) served as negative controls. At the end of 6 days cells were stained on the surface for CD3, CD8, and intracellular Ki-67, acquired on a FACSCalibur, and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). CD4 cells (CD3+, CD8−) were analyzed for CFSE dilution and Ki-67 expression. The numbers on the plots represent the frequency of CFSE−, Ki-67+ cells as a percentage of total CD4 T cells. (C) Summary of proliferation data for SIV-infected macaques. Fold increase (frequency of CFSE-negative, Ki-67-positive cells in stimulated cultures over unstimulated cultures) in the frequency of Gag-specific cells is plotted for each macaque. Each symbol represents an individual macaque.
Similar articles
- Vaccine-induced, simian immunodeficiency virus-specific CD8+ T cells reduce virus replication but do not protect from simian immunodeficiency virus disease progression.
Engram JC, Dunham RM, Makedonas G, Vanderford TH, Sumpter B, Klatt NR, Ratcliffe SJ, Garg S, Paiardini M, McQuoid M, Altman JD, Staprans SI, Betts MR, Garber DA, Feinberg MB, Silvestri G. Engram JC, et al. J Immunol. 2009 Jul 1;183(1):706-17. doi: 10.4049/jimmunol.0803746. J Immunol. 2009. PMID: 19542473 - Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses.
Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Hel Z, et al. J Immunol. 2002 Nov 1;169(9):4778-87. doi: 10.4049/jimmunol.169.9.4778. J Immunol. 2002. PMID: 12391187 - Vaccination of Macaques with DNA Followed by Adenoviral Vectors Encoding Simian Immunodeficiency Virus (SIV) Gag Alone Delays Infection by Repeated Mucosal Challenge with SIV.
Almond N, Berry N, Stebbings R, Preston M, Ham C, Page M, Ferguson D, Rose N, Li B, Mee ET, Hassall M, Stahl-Hennig C, Athanasopoulos T, Papagatsias T, Herath S, Benlahrech A, Dickson G, Meiser A, Patterson S. Almond N, et al. J Virol. 2019 Oct 15;93(21):e00606-19. doi: 10.1128/JVI.00606-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413132 Free PMC article. - Cytotoxic T lymphocytes specific for the simian immunodeficiency virus.
Letvin NL, Schmitz JE, Jordan HL, Seth A, Hirsch VM, Reimann KA, Kuroda MJ. Letvin NL, et al. Immunol Rev. 1999 Aug;170:127-34. doi: 10.1111/j.1600-065x.1999.tb01334.x. Immunol Rev. 1999. PMID: 10566147 Review.
Cited by
- Immune restoration by TIGIT blockade is insufficient to control chronic SIV infection.
Webb GM, Pessoa CT, McCullen AJ, Hwang JM, Humkey MC, Thormin-Odum R, Kukula KA, Smedley J, Fischer M, Sciurba J, Bochart RM, Shriver-Munsch C, Ndhlovu LC, Sacha JB. Webb GM, et al. J Virol. 2024 Jun 13;98(6):e0027324. doi: 10.1128/jvi.00273-24. Epub 2024 May 22. J Virol. 2024. PMID: 38775481 - Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection.
Mu W, Patankar V, Kitchen S, Zhen A. Mu W, et al. Viruses. 2024 Jan 31;16(2):219. doi: 10.3390/v16020219. Viruses. 2024. PMID: 38399994 Free PMC article. Review. - Changes to the Simian Immunodeficiency Virus (SIV) Reservoir and Enhanced SIV-Specific Responses in a Rhesus Macaque Model of Functional Cure after Serial Rounds of Romidepsin Administrations.
Kleinman AJ, Sivanandham S, Sette P, Sivanandham R, Policicchio BB, Xu C, Penn E, Brocca-Cofano E, Le Hingrat Q, Ma D, Pandrea I, Apetrei C. Kleinman AJ, et al. J Virol. 2022 Jun 22;96(12):e0044522. doi: 10.1128/jvi.00445-22. Epub 2022 May 31. J Virol. 2022. PMID: 35638831 Free PMC article. - Lymph node CXCR5+ NK cells associate with control of chronic SHIV infection.
Rahman SA, Billingsley JM, Sharma AA, Styles TM, Govindaraj S, Shanmugasundaram U, Babu H, Riberio SP, Ali SA, Tharp GK, Ibegbu C, Waggoner SN, Johnson RP, Sekaly RP, Villinger F, Bosinger SE, Amara RR, Velu V. Rahman SA, et al. JCI Insight. 2022 Apr 22;7(8):e155601. doi: 10.1172/jci.insight.155601. JCI Insight. 2022. PMID: 35271506 Free PMC article. - Diagnostic Performance of PD-L1 versus PD-1 Expression in Circulating CD20 Cells in Diffuse Large B-Cell Lymphoma.
Saber MM. Saber MM. Antibodies (Basel). 2022 Feb 16;11(1):15. doi: 10.3390/antib11010015. Antibodies (Basel). 2022. PMID: 35225873 Free PMC article.
References
- Akbar, A. N., N. J. Borthwick, R. G. Wickremasinghe, P. Panayoitidis, D. Pilling, M. Bofill, S. Krajewski, J. C. Reed, and M. Salmon. 1996. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294-299. - PubMed
- Amara, R. R., K. Patel, G. Niedziela, P. Nigam, S. Sharma, S. I. Staprans, D. C. Montefiori, L. Chenareddi, J. G. Herndon, H. L. Robinson, H. M. McClure, and F. J. Novembre. 2005. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides enhanced protection from simian/human immunodeficiency virus-induced disease. J. Virol. 79:15356-15367. - PMC - PubMed
- Amara, R. R., J. M. Smith, S. Staprans, D. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol for the control of a pathogenic SHIV challenge by a DNA/rMVA vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
- Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 RR00165/RR/NCRR NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- R01 AI057029/AI/NIAID NIH HHS/United States
- P30 AI50409/AI/NIAID NIH HHS/United States
- R01 HL075833/HL/NHLBI NIH HHS/United States
- P01 AI049364/AI/NIAID NIH HHS/United States
- P01 AI49364/AI/NIAID NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- R01 AI57029/AI/NIAID NIH HHS/United States
- AI56299/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials