Dissection of double-stranded RNA binding protein B2 from betanodavirus - PubMed (original) (raw)
Dissection of double-stranded RNA binding protein B2 from betanodavirus
Beau J Fenner et al. J Virol. 2007 Jun.
Abstract
Betanodaviruses are small RNA viruses that infect teleost fish and pose a considerable threat to marine aquaculture production. These viruses possess a small protein, termed B2, which binds to and protects double-stranded RNA. This prevents cleavage of virus-derived double-stranded RNAs (dsRNAs) by Dicer and subsequent production of small interfering RNA (siRNA), which would otherwise induce an RNA-silencing response against the virus. In this work, we have performed charged-to-alanine scanning mutagenesis of the B2 protein in order to identify residues required for dsRNA binding and protection. While the majority of the 19 mutated B2 residues were required for maximal dsRNA binding and protection in vitro, residues R53 and R60 were essential for both activities. Subsequent experiments in fish cells confirmed these findings by showing that mutations in these residues abolished accumulation of both the RNA1 and RNA2 components of the viral genome, in addition to preventing any significant induction of the host interferon gene, Mx. Moreover, an obvious positive correlation was found between dsRNA binding and protection in vitro and RNA1, RNA2, and Mx accumulation in fish cells, further validating the importance of the selected amino acid residues. The same trend was also demonstrated using an RNA silencing system in HeLa cells, with residues R53 and R60 being essential for suppression of RNA silencing. Importantly, we found that siRNA-mediated knockdown of Dicer dramatically enhanced the accumulation of a B2 mutant. In addition, we found that B2 is able to induce apoptosis in fish cells but that this was not the result of dsRNA binding.
Figures
FIG. 1.
Cleavage of virus-derived dsRNA by RNase III and protection by GGNNV B2. (A) Fixed quantities of purified GST-B2 were incubated with dsRNA in the presence of various amounts of RNase III, and the products were resolved by nondenaturing PAGE. Control reactions using GST are shown on the right. (B) Band quantitation of the image shown in panel A, with values shown as arbitrary units (AU). Increasing amounts of added RNase III resulted in a greater level of dsRNA digestion, though only 0.03 U of RNase III was required for complete digestion in the absence of recombinant B2. See Materials and Methods for experimental details.
FIG. 2.
Charged-to-alanine scanning mutagenesis of GGNNV B2. (A) Amino acid sequence of B2, with charged residues targeted for mutagenesis into alanine in boldface. (B) SDS-PAGE of purified GST-B2 mutants. Marker sizes are indicated on the left.
FIG. 3.
The dsRNA binding and protection activity of B2 is severely impaired by charged-to-alanine mutagenesis. (A) EMSA analysis of dsRNA binding by the mutant B2 proteins. A previously described (11) 40-bp dsRNA target at a concentration of 0.1 μM was incubated with GST (negative control), GST-B2 (wild type), or the mutant B2 proteins at 1 μM concentrations, and the products were separated by nondenaturing PAGE. The resulting mobility shift of the dsRNA was taken as a measure of the dsRNA affinity of the proteins. (B) Protection of long dsRNA by B2 and its mutants against RNase III digestion. (C) Quantitative analysis of the EMSA and RNase III protection results shown in panels B and C. Values obtained using wild-type B2 were normalized to 100%, with mutant protein values expressed relative to the wild type. (D) Correlation between 40-bp dsRNA binding and RNase III protection data for B2 mutants. Values shown in panel C were plotted as an XY scatter plot, and linear regression was calculated for the pool of mutants. Mutants selected for further analyses are indicated by stars. (E) Alignment of betanodavirus B2 proteins showing the conservation of important amino acid residues required for dsRNA binding and protection. Identical residues are indicated by asterisks, and dsRNA binding-related residues are boxed.
FIG. 4.
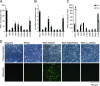
GGNNV depends on the dsRNA binding activity of B2 for accumulation in SB cells. (A) Accumulation of RNA1 and its B2 mutants in SB cells. Subconfluent SB cells were cotransfected with 100 ng RNA1 or its B2 mutant derivatives, and RNA1 was measured by qRT-PCR at 48 h posttransfection. (B) Induction of the SB Mx gene by RNA1 or its B2 mutant derivatives. RNA samples were the same as for panel A. (C) RNA1-dependent accumulation of RNA2 in SB cells and the influence of B2 mutations on this process. RNA was prepared from SB cells at 48 h (filled bars) or 72 h (empty bars) posttransfection, and RNA2 was measured by qRT-PCR. Bars represent the means of at least three independent experiments, with the standard deviation indicated by error bars. (D) Mutation of GGNNV B2 attenuates viral pathogenicity and production of the alpha coat protein. Subconfluent SB cells were cotransfected with 100 ng of RNA2 and RNA1 or its B2 mutant derivatives. Mutant E2A is shown as an example, though the remaining mutants yielded results that were indistinguishable from those of this mutant (data not shown). Examples of cytopathic effects visible in cells cotransfected with RNA1/RNA2 are indicated with arrowheads. The upper row of images shows phase-contrast images of the transfected cells, while the lower row shows the same cells stained for alpha coat protein using immunofluorescence.
FIG. 5.
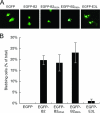
Cell death caused by B2 overexpression is not related to dsRNA binding. (A) SB cell blebbing is evident after transfection with an EGFP-B2 plasmid or its dsRNA binding mutants, but not after transfection with EGFP or EGFP-E3L plasmids. Subconfluent SB cells were transfected with 2 μg of each plasmid, and representative healthy or blebbing cells were photographed at 72 h. (B) Counts of blebbing SB cells from the experiment described in panel A. A minimum of 300 transfected (green) SB cells were counted and scored for blebbing. The results are expressed as a percentage of the total cells counted, with the error bars representing the standard deviation from three independent experiments.
FIG. 6.
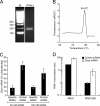
A B2 mutant of RNA1 cannot accumulate in HeLa cells due to the action of the Dicer RNase. (A) Amplification of Dicer_Hs_ mRNA by qRT-PCR. Total RNA from HeLa cells was extracted and subjected to RT-PCR as described in Materials and Methods. The amplicon size is 165 bp, corresponding to the observed band. (B) Melting curve analysis of the Dicer_Hs_ qRT-PCR product. A single peak with an apparent melting temperature of 84.4°C was obtained. (C) RNA silencing of Dicer_Hs_ mRNA in HeLa cells by siRNA transfection. Cells were left untransfected or were transfected with 10 or 50 pmol of a control siRNA or a specific siRNA. Dicer_Hs_ mRNA was quantitated after 48 h by qRT-PCR. Values are expressed as a function of the amount of Dicer_Hs_ mRNA present in untransfected HeLa cells. (D) Influence of siRNA-mediated Dicer knockdown on RNA1 and RNA1ΔB2 accumulation in transfected HeLa cells. Cells were transfected as described in Materials and Methods, and RNA1 was measured by qRT-PCR. Values shown are the means of three independent determinations, with the error bars indicating the standard deviations.
FIG. 7.
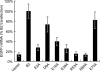
The ability of B2 to suppress RNA silencing in HeLa cells is dependent on its dsRNA binding activity. HeLa cell transfections were performed using either pcDNA3.1(+) (vector) or pcDNA-B2 and its mutants, pEGFP-C1, and anti-EGFP shRNA vectors as described previously (12). Values shown are percentages of the amount of EGFP mRNA detected by qRT-PCR in the pcDNA-B2 transfection, with error bars indicating the standard deviation of at least three independent determinations.
FIG. 8.
Charged amino acid mutations induce minor secondary structural changes to GGNNV B2 based on structural modeling. Comparisons of (A) FHV B2 and (B) Staufen dsRBD hypothetical (Hyp.) and experimental (Exp.) structures are shown in the top two panels, with alpha-helical regions indicated by a red α, beta sheets indicated by a blue β, and random coils indicated by dashes. Matches of 89% (64/72) and 85% (58/68) between hypothetical and experimental structures were obtained for FHV B2 and Staufen dsRBD, respectively. (C) GGNNV B2 is predicted to have a largely alpha-helical structure that is susceptible to minor structural changes caused by charged-to-alanine mutagenesis. A secondary structure model of wild-type (WT) B2 is shown aligned to models of each of the chosen mutants, with conserved positions shown as centered dots. The positions of each mutation are shown as asterisks. Modeling was performed using the PSIPRED method (20, 31).
Similar articles
- Dual modes of RNA-silencing suppression by Flock House virus protein B2.
Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR. Chao JA, et al. Nat Struct Mol Biol. 2005 Nov;12(11):952-7. doi: 10.1038/nsmb1005. Nat Struct Mol Biol. 2005. PMID: 16228003 - Identification of critical residues in nervous necrosis virus B2 for dsRNA-binding and RNAi-inhibiting activity through by bioinformatic analysis and mutagenesis.
Ou MC, Chen YM, Jeng MF, Chu CJ, Yang HL, Chen TY. Ou MC, et al. Biochem Biophys Res Commun. 2007 Sep 28;361(3):634-40. doi: 10.1016/j.bbrc.2007.07.075. Epub 2007 Jul 24. Biochem Biophys Res Commun. 2007. PMID: 17669362 - Sequestration and protection of double-stranded RNA by the betanodavirus b2 protein.
Fenner BJ, Goh W, Kwang J. Fenner BJ, et al. J Virol. 2006 Jul;80(14):6822-33. doi: 10.1128/JVI.00079-06. J Virol. 2006. PMID: 16809288 Free PMC article. - The double-stranded-RNA-binding motif: interference and much more.
Tian B, Bevilacqua PC, Diegelman-Parente A, Mathews MB. Tian B, et al. Nat Rev Mol Cell Biol. 2004 Dec;5(12):1013-23. doi: 10.1038/nrm1528. Nat Rev Mol Cell Biol. 2004. PMID: 15573138 Review. - Double-stranded RNA binding proteins and their substrates.
Bass BL. Bass BL. Nucleic Acids Symp Ser. 1995;(33):13-5. Nucleic Acids Symp Ser. 1995. PMID: 8643348 Review.
Cited by
- Antiviral immunity directed by small RNAs.
Ding SW, Voinnet O. Ding SW, et al. Cell. 2007 Aug 10;130(3):413-26. doi: 10.1016/j.cell.2007.07.039. Cell. 2007. PMID: 17693253 Free PMC article. Review. - RNA binding by a novel helical fold of b2 protein from wuhan nodavirus mediates the suppression of RNA interference and promotes b2 dimerization.
Qi N, Cai D, Qiu Y, Xie J, Wang Z, Si J, Zhang J, Zhou X, Hu Y. Qi N, et al. J Virol. 2011 Sep;85(18):9543-54. doi: 10.1128/JVI.00785-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734038 Free PMC article. - Protease activity, self interaction, and small interfering RNA binding of the silencing suppressor p1b from cucumber vein yellowing ipomovirus.
Valli A, Dujovny G, García JA. Valli A, et al. J Virol. 2008 Jan;82(2):974-86. doi: 10.1128/JVI.01664-07. Epub 2007 Nov 7. J Virol. 2008. PMID: 17989179 Free PMC article. - Small Non-coding RNAs Associated with Viral Infectious Diseases of Veterinary Importance: Potential Clinical Applications.
Samir M, Pessler F. Samir M, et al. Front Vet Sci. 2016 Apr 4;3:22. doi: 10.3389/fvets.2016.00022. eCollection 2016. Front Vet Sci. 2016. PMID: 27092305 Free PMC article. Review. - Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review.
Sudhagar A, Kumar G, El-Matbouli M. Sudhagar A, et al. Int J Mol Sci. 2018 Jan 15;19(1):245. doi: 10.3390/ijms19010245. Int J Mol Sci. 2018. PMID: 29342931 Free PMC article. Review.
References
- Bagasra, O., and K. R. Prilliman. 2004. RNA interference: the molecular immune system. J. Mol. Histol. 35:545-553. - PubMed
- Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. - PubMed
- Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources