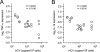Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients - PubMed (original) (raw)
Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients
Ian Gaël Rodrigue-Gervais et al. J Virol. 2007 Jun.
Abstract
The role of peripheral dendritic cells (DCs) in hepatitis C virus (HCV) infection is unclear. To determine if persistent infection exerts an inhibitory pressure on HCV-specific innate responses, we analyzed DC function in blood through quantification of cell-associated HCV RNA levels in conjunction with multiparametric flow cytometry analysis of pathogen recognition receptor-induced cytokine expression. Independently of the serum viral load, fluorescence-activated cell sorter-purified total DCs had a wide range of cell-associated HCV genomic RNA copy numbers (mean log(10), 5.0 per 10(6) cells; range, 4.3 to 5.8). Here we report that for viremic patients with high viral loads in their total DCs, the myeloid DC (MDC) subset displayed impaired expression of interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-alpha) but normal IL-6 or chemokine CCL3 expression in response to poly(I:C) and lipopolysaccharide (LPS). IL-6-expressing cells from this subgroup of viremic patients demonstrated a significant increase (sixfold more) in TNF-alpha(-) IL-12(-) cell frequency compared to healthy donors (mean, 38.8% versus 6.5%; P < 0.0001), indicating a functional defect in a subpopulation of cytokine-producing MDCs ( approximately 6% of MDCs). Attenuation of poly(I:C) and LPS innate sensing was HCV RNA density dependent and did not correlate with viremia or deficits in circulating MDC frequencies in HCV-infected patients. Monocytes from these patients were functionally intact, responding normally on a per-cell basis following stimulation, independent of cell-associated HCV RNA levels. Taken together, these data indicate that detection of HCV genomic RNA in DCs and loss of function in the danger signal responsiveness of a small proportion of DCs in vivo are interrelated rather than independent phenomena.
Figures
FIG. 1.
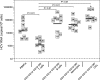
HCV RNA contents in circulating immune blood cell subpopulations from viremic patients. HCV sense-strand RNA copy numbers in FACS-purified blood T cells (CD3+), B cells (CD19+), monocytes (CD14+), and total DCs (CD3− CD14− CD19− HLA-DR+) from nine viremic patients were quantified by LightCycler hybridization probe technology. The data are the averaged values for replicates from two independent measurements expressed as absolute numbers of HCV RNA molecules per 106 cells. Each square indicates the detection of viral RNA from a patient, with the corresponding identification number inside the square. The solid horizontal lines represent the average for each group. Differences between T cells and other subsets, as calculated by one-way ANOVA with Tukey's test, are shown above the graph. *, P < 0.01 for DCs versus PBMCs.
FIG. 2.
MDCs display impaired response to poly(I:C) and LPS in viremic patients. PBMCs were cultured for 1 h in the absence or presence of poly(I:C) or LPS before the addition of brefeldin A (10 μg/ml) for a further 5 h. Cells were recovered and stained for the indicated intracellular proteins. (A) (Left) Heat map indicating log2 changes in IL-6, IL-12, TNF-α, and CCL3 protein expression profiles for CD14− CD33+ MHC-IIbr MDCs from patients, treated with poly(I:C) or LPS for 6 h, versus protein expression in the same population from healthy donors. Expression signatures for 7 healthy blood donors (D01 to D07), 2 resolvers (SR-P31 and SVR-P35), and 14 viremic (HCV) patients were grouped according to similarity, using the complete linkage hierarchical clustering algorithm in MeV software (left margin). Ratios of the different fluorescent intracellular antibodies (columns) were determined by calculating log2(MFIstimulated/MFIunstimulated) and subtracting healthy donor sample means. Each square in the grid corresponds to a flow cytometry file containing approximately 5,000 CD14− CD33+ MHC-IIbr cell events. Protein expression changes relative to healthy donors are indicated by the color intensity scale (red, more expression than that of healthy donors; blue, less expression than that of healthy donors; and white, no change relative to healthy donors). SB202190 (10 μM for 30 min prior to stimulation) served as a positive control for down-regulation of the analyzed inflammatory mediators. (Right) Association between HCV RNA abundance in PBMCs (black bars), FACS-purified total DCs (CD3− CD19− CD14− MHC-II+; white bars), and cytokine expression profiles (CP). The asterisk indicates that HCV quantification was not done due to an unavailability of cell samples. The solid vertical line at 105 on the x axis represents a reference point. Statistical analysis was done by Fisher's two-tailed exact probability test on DCs (P = 0.004). (B) Groupwise comparisons between healthy donors and CP-N and CP-D subjects. IL-12, TNF-α, and IL-6 increases above baseline (_x_-fold) (MFIstimulated/MFIunstimulated) are shown for each cluster. Combined data for poly(I:C) and LPS responses and averages (solid horizontal lines) are shown. Statistical comparisons between groups were calculated by one-way ANOVA with Tukey's test.
FIG. 3.
Poly(I:C) and LPS responsiveness in MDCs is associated with HCV RNA levels in total DCs. Dot plots show TNF-α (A) and IL-12 (B) responses (log2) as a function of HCV RNA copy numbers in FACS-purified DCs. Data points (two per patient) represent the mean levels of cytokine expression as log2 changes versus those of the donors for two independent measurements. A negative correlation was observed for HCV-infected patients between HCV RNA and IL-12 or TNF-α expression (n = 10). Correlation statistics were analyzed using the Spearman rank correlation test.
FIG. 4.
Selective attenuation of poly(I:C) and LPS innate sensing in IL-6-expressing MDCs of CP-D viremic patients. (A) Flow cytometry plots of IL-6 (x axis [top row]), TNF-α, and IL-12 (y and x axes, respectively [bottom row]) expression following stimulation in two representative patient samples (of 16), namely, HCV-P28 and HCV-P29. Histograms are gated on total MDCs (gate I) or monocytes (gate II), as shown in panel B. Arrowheads in dot plots indicate a small population of bright cells that was absent from P29. Numbers in bottom right corners and above bracketed lines indicate the percentages of cells in the designated areas. (B) CD14− CD33+ MHC-IIbr (gate I) and CD14+ CD33+ MHC-II+ (gate II) populations used for FACS analysis in the experiments shown in panel A. (C) Frequencies of IL-6+ MDCs positive for TNF-α and IL-12 for all patients analyzed by FACS after poly(I:C) (triangles) and LPS (circles) activation. Groupwise comparisons between healthy donors (n = 7) and CP-N (n = 8) and CP-D (n = 8) subjects were determined with the Mann-Whitney rank sum test and are shown at the top of the graph.
FIG. 5.
Influence of HCV on peripheral blood DC subset frequencies. Freshly isolated uncultured PBMCs (day 0) were surface stained for eight-color multiparametric flow cytometry. (A and B) Flow cytometry dot plots representative of circulating frequencies of CD3− CD19− gated PDCs (A) and MDCs (B) are shown for healthy volunteers (D03 [top]) and HCV-infected patients (P20 [bottom]). Gate 1 corresponds to CD303+ CD123br CD4hi CD11c− CD14− CD62L+ HLA-DR+ PDCs, and gate 4 corresponds to CD11c+ CD14− CD16− CD4+ CD123dim CD62L+ HLA-DRbr MDCs. In gate 3, CD123+ CD303− cells, which correspond to basophilic granulocytes, did not stain for HLA-DR, and gate 2 shows CD303− CD16+ NK lymphocytes and monocytes. For each DC gate, the fraction of cells relative to the total number of PBMCs is indicated as a percentage. (C) Box-and-whisker-plot representation of circulating frequencies of CD303+ PDCs and CD11c+ MDCs in HCV-infected individuals (gray boxes; n = 13) and healthy age-matched controls (white boxes; n = 7), measured as percentages of total PBMCs collected after Ficoll separation. The ends of the boxes define the 25th and 75th percentiles, a horizontal line indicates the median, and bars define the 5th and 95th percentiles. P values were determined with the Mann-Whitney rank sum test and are shown at the top of the graph (HCV+ versus HCV−). (D and E) There is no correlation between TNF-α and IL-12 cytokine production potentials and MDC frequencies in HCV-infected patients. Correlation statistics were analyzed using the Spearman rank correlation test.
Similar articles
- Monocyte-derived dendritic cells from chronic HCV patients are not infected but show an immature phenotype and aberrant cytokine profile.
Gelderblom HC, Nijhuis LE, de Jong EC, te Velde AA, Pajkrt D, Reesink HW, Beld MG, van Deventer SJ, Jansen PL. Gelderblom HC, et al. Liver Int. 2007 Sep;27(7):944-53. doi: 10.1111/j.1478-3231.2007.01507.x. Liver Int. 2007. PMID: 17696933 - Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection.
Rodrigue-Gervais IG, Rigsby H, Jouan L, Sauvé D, Sékaly RP, Willems B, Lamarre D. Rodrigue-Gervais IG, et al. J Immunol. 2010 Mar 15;184(6):3134-44. doi: 10.4049/jimmunol.0902522. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173023 - Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I.
Miyazaki M, Kanto T, Inoue M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kakita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Miyazaki M, et al. J Med Virol. 2008 Jun;80(6):980-8. doi: 10.1002/jmv.21174. J Med Virol. 2008. PMID: 18428149 - [Hepatitis C virus subverts pattern recognition receptors-mediated control of adaptative immunity orchestrated by dendritic cells].
Rodrigue-Gervais IG, Lamarre D. Rodrigue-Gervais IG, et al. Med Sci (Paris). 2010 Oct;26(10):869-74. doi: 10.1051/medsci/20102610869. Med Sci (Paris). 2010. PMID: 20929679 Review. French. - The affect of chronic hepatitis C infection on dendritic cell function: a summary of the experimental evidence.
Ryan EJ, O'Farrelly C. Ryan EJ, et al. J Viral Hepat. 2011 Sep;18(9):601-7. doi: 10.1111/j.1365-2893.2011.01453.x. Epub 2011 Mar 14. J Viral Hepat. 2011. PMID: 21794024 Review.
Cited by
- Reversing immune dysfunction and liver damage after direct-acting antiviral treatment for hepatitis C.
Mazouz S, Boisvert M, Shoukry NH, Lamarre D. Mazouz S, et al. Can Liver J. 2018 Jul 17;1(2):78-105. doi: 10.3138/canlivj.1.2.007. eCollection 2018 Spring. Can Liver J. 2018. PMID: 35990715 Free PMC article. Review. - Natural killer cells: multifaceted players with key roles in hepatitis C immunity.
Golden-Mason L, Rosen HR. Golden-Mason L, et al. Immunol Rev. 2013 Sep;255(1):68-81. doi: 10.1111/imr.12090. Immunol Rev. 2013. PMID: 23947348 Free PMC article. Review. - Th1 disabled function in response to TLR4 stimulation of monocyte-derived DC from patients chronically-infected by hepatitis C virus.
Perrin-Cocon L, Agaugué S, Diaz O, Vanbervliet B, Dollet S, Guironnet-Paquet A, André P, Lotteau V. Perrin-Cocon L, et al. PLoS One. 2008 May 28;3(5):e2260. doi: 10.1371/journal.pone.0002260. PLoS One. 2008. PMID: 18509450 Free PMC article. - A look behind closed doors: interaction of persistent viruses with dendritic cells.
Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. Lambotin M, et al. Nat Rev Microbiol. 2010 May;8(5):350-60. doi: 10.1038/nrmicro2332. Epub 2010 Apr 7. Nat Rev Microbiol. 2010. PMID: 20372157 Free PMC article. Review. - Regulation of host innate immunity by hepatitis C virus: crosstalk between hepatocyte and NK/DC.
Park SJ, Hahn YS. Park SJ, et al. Rev Infect. 2010 Jul 1;1(3):151-157. Rev Infect. 2010. PMID: 24688607 Free PMC article.
References
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
- Anthony, D. D., N. L. Yonkers, A. B. Post, R. Asaad, F. P. Heinzel, M. M. Lederman, P. V. Lehmann, and H. Valdez. 2004. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J. Immunol. 172:4907-4916. - PubMed
- Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. - PubMed
- Boisvert, J., X. S. He, R. Cheung, E. B. Keeffe, T. Wright, and H. B. Greenberg. 2001. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J. Infect. Dis. 184:827-835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources