Maturation of ribbon synapses in hair cells is driven by thyroid hormone - PubMed (original) (raw)
Comparative Study
Maturation of ribbon synapses in hair cells is driven by thyroid hormone
Gaston Sendin et al. J Neurosci. 2007.
Abstract
Ribbon synapses of inner hair cells (IHCs) undergo developmental maturation until after the onset of hearing. Here, we studied whether IHC synaptogenesis is regulated by thyroid hormone (TH). We performed perforated patch-clamp recordings of Ca2+ currents and exocytic membrane capacitance changes in IHCs of athyroid and TH-substituted Pax8-/- mice during postnatal development. Ca2+ currents remained elevated in athyroid IHCs at the end of the second postnatal week, when it had developmentally declined in wild-type and TH-rescued mutant IHCs. The efficiency of Ca2+ influx in triggering exocytosis of the readily releasable vesicle pool was reduced in athyroid IHCs. Ribbon synapses were formed despite the TH deficiency. However, different from wild type, in which synapse elimination takes place at approximately the onset of hearing, the number of ribbon synapses remained elevated in 2-week-old athyroid IHCs. Moreover, the ultrastructure of these synapses appeared immature. Using quantitative reverse transcription-PCR, we found a TH-dependent developmental upregulation of the mRNAs for the neuronal SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, SNAP25 (synaptosomal-associated protein of 25 kDa) and synaptobrevin 1, in the organ of Corti. These molecular changes probably contribute to the improvement of exocytosis efficiency in mature IHCs. IHCs of 2-week-old athyroid Pax8-/- mice maintained the normally temporary efferent innervation. Moreover, they lacked large-conductance Ca2+-activated K+ channels and KCNQ4 channels. This together with the persistently increased Ca2+ influx permitted continued action potential generation. We conclude that TH regulates IHC differentiation and is essential for morphological and functional maturation of their ribbon synapses. We suggest that presynaptic dysfunction of IHCs is a mechanism in congenital hypothyroid deafness.
Figures
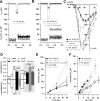
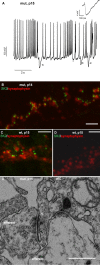
Figure 1.
Impaired maturation of presynaptic function in IHCs of _Pax8_−/− mice. A and B plot representative Ca2+ current traces (_I_Ca; top) and exocytic capacitance responses (Δ_C_m; bottom) for P7 (A) and P15 (B) mut (gray), rescued mut (dark gray), and wt (black) IHCs elicited by step depolarization (50 ms) to −14 mV (peak Ca2+ current potential). The _R_s values were as follows: 18.1 MΩ for wt P7, 20.1 MΩ for mut P7, 16.4 MΩ for wt P15, 20.8 MΩ for untreated mut P15, and 17.4 MΩ for TH-treated mut P15. In C, average current–voltage relationships recorded from wt (n = 11 for P6–P8; n = 9 for P14–P16) and _Pax8_−/− IHCs (athyroid, n = 7 IHCs for P6–P8 and n = 7 IHCs for P14–P16; rescued, n = 3 for P14–P15) are displayed. D shows developmental changes of ΔCm (top) and Ca2+ current integral (_Q_Ca; bottom) in response to short depolarizations in IHCs from NMRI mice (white bars) (data from Beutner and Moser, 2001), Pax8+/+ (or C57BL/6; black, data as in E, F), and _Pax8_−/− mice without (gray) and with (dark gray) TH substitution. All data have been normalized to the P14–P16 Pax8+/+ results. E, F, Δ_C_m to depolarizations to the peak Ca2+ current potential having variable duration were recorded for wt (n = 12 for P6–P8, n = 12 for P14–P16) and _Pax8_−/− (athyroid, n = 7 for P6–P8 and n = 7 for P14–P16; n = 3 for TH-rescued P14–P16) IHCs were plotted versus the stimulus duration (E). F displays binned Δ_C_m data versus their corresponding _Q_Ca of P6–P8 and P14–P16 wt and mut (untreated) IHCs. Intervals between the pulses were at least 30 s to allow for complete recovery of the readily releasable pool (Moser and Beutner, 2000).
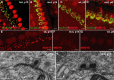
Figure 2.
IHC ribbon synapses are morphologically immature in athyroid _Pax8_−/− mice at p15. A–D, Representative projections of confocal sections obtained from P15 heterozygote (A), athyroid P14 mut (B), P8 wt (C), and P6 athyroid mut (D) organs of Corti stained for RIBEYE/CtBP2 (red) and GluR2/3 (green). Synaptic ribbons were identified as small RIBEYE-positive spots juxtaposing GluR2/3 immunofluorescence spots in P15 wt IHCs (A). GluR2/3 immunofluorescence was less confined in athyroid P6 and P14 mut as well as P8 wt IHCs. E–G, Red channel only, of comparable projections as used for counts of small RIBEYE-positive spots in P15 wt (E), P14 athyroid mut (F), and P8 wt (G) organs of Corti. In A–C and E, specimens were fixed with 4% paraformaldehyde at room temperature. In D, F, and G, MetOH (99%) at −20°C was used. H, I, Electron micrographs of IHC ribbon synapses from P15 wt (H) and P15 athyroid mut (I) mice. Synaptic ribbons are seen as electron-dense bodies, each with a halo of synaptic vesicles and positioned close to the presynaptic membrane, opposing the postsynaptic density of the afferent fiber (aff). I shows an active zone representative for its large extension holding two ribbons and showing additional small vesicle clusters (asterisks). The arrowhead points toward a probably endocytic membrane invagination. In addition to the synaptic vesicles (small vesicles with rather homogeneous size), cisternae and tubes of varying size and shape as well as mitochondria were observed. Scale bars: A–G, 5 μm; H, I, 200 nm.
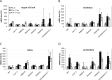
Figure 3.
Molecular changes accompanying postnatal maturation of IHC presynaptic morphology and function. A–D show relative amounts of otoferlin, RIBEYE, Cav1.3, Bassoon, SNAP25, and synaptobrevin 1 mRNA estimated by quantitative RT-PCR. Total RNA was isolated from organ of Corti (A), modiolus (B), retina (C), and cerebellum (D) of P6 Pax8+/+, P14–P17 Pax8+/+, and P14–P17 _Pax8_−/− mice (n = 12 mice each). The expression of the target mRNAs was normalized first to that of TBP and then to the values obtained for corresponding mRNA and tissue of P6 Pax8+/+ mice. The data are represented as means from four independent experiments in which all samples were analyzed in triplicates (error bars indicate 95% confidence intervals of the population estimate of mRNA abundance).
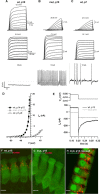
Figure 4.
Lack of large-conductance Ca2+-activated K+ currents and KCNMA1 immunoreactivity: persistence of action potentials. A–C display representative membrane currents (top two rows) and potentials (bottom row) of P15 wt and _Pax8_−/− as well as P7 wt IHCs. Top row, Three millisecond depolarizations (maximum potential indicated) elicited fast outward currents (BK currents) in this P15 wt IHC but in neither P15 mut nor P7 wt IHCs, which showed slower activation of delayed rectifier currents only. _R_s values were as follows: 4.92, 4.48, and 2.48 MΩ for wt P15, mut P15, and wt P7, respectively. Middle row, Large currents in all three groups in response to 100 ms voltage steps but lack of BK current in mut and wt P7 IHCs. _R_s values were as follows: 3.16, 4.27, and 3.96 MΩ for wt P15, mut P15, and wt P7, respectively. Bottom row, Membrane potentials in response to injection of 50 pA (P15 wt) and 10 pA (P15 mut and P7 wt) depolarizing current. Action potentials were observed in P15 mut and P7 wt IHCs but not in P15 wt despite stronger current injection. D, Mean I–V relationship for 3 ms depolarizations, obtained by averaging currents for 1 ms (starting 1 ms after stimulus onset) in IHCs of P14–P16 wt (black squares; n = 6) and _Pax8_−/− (gray squares; n = 4) as well as P6–P8 wt IHCs (circles; n = 6). E, Representative currents of a P15 wt IHC (black) and a P15 mut IHC (gray) during repolarization to −154 mV from −64 mV (200 ms), clearly showing a deactivating current characteristic for KCNQ4 channels in the wt but not in the mut IHC; no P/n correction was applied. _R_s values were as follows: 2.49 and 5.24 MΩ for wt P15 and mut P15. F–H show representative projections of confocal sections obtained from organs of Corti of P15 WT (F), mut (G), and rescued mut (H) mice after KCNMA1 (red) and parvalbumin (green) immunostaining: note spots of KCNMA1 immunofluorescence at the neck of wt and rescued mut IHCs, which are lacking in athyroid mut IHCs. PFA (4%) was used as fixative. Scale bars, 5 μm.
Figure 5.
Prolonged presence of efferent IHC synapses in _Pax8_−/− mice. A shows a membrane potential recording from a P15 _Pax8_−/− IHC displaying spontaneous action potentials and small biphasic potentials (arrowheads; example enlarged in the inset) characteristic for cholinergic postsynaptic potentials. B–D show representative projections of confocal sections obtained from organs of Corti of a P15 mut (B) and a P15 wt (C, D) mice after synaptophysin (red) and SK2 (green) immunostaining. E, Representative electron micrograph showing the basal pole of a P15 mut IHC with an efferent synapse onto the IHC featuring the presynaptic terminal with synaptic vesicles and the characteristic postsynaptic cistern in the IHC (arrowheads). The IHC forms a ribbon synapse with an afferent fiber next to the efferent IHC synapse. Scale bars: B–D, 2 μm; E, 500 nm.
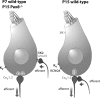
Figure 6.
Schematic representation of basolateral IHC properties before and after the onset of hearing in wt and in 2-week-old TH-deficient _Pax8_−/− mice. A sketches the immature IHC morphological and functional properties found in wt IHCs before the onset of hearing and in 2-week-old _Pax8_−/− IHCs. B, Mature basolateral makeup of IHCs after the onset of hearing. For simplicity, we only drew one afferent and efferent synapse each (at the same size) and did not depict the branching of afferent fibers seen in immature organs of Corti. Structures (ion channels and synapses) are not drawn to scale.
Similar articles
- Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells.
Brandt N, Kuhn S, Münkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Brandt N, et al. J Neurosci. 2007 Mar 21;27(12):3174-86. doi: 10.1523/JNEUROSCI.3965-06.2007. J Neurosci. 2007. PMID: 17376979 Free PMC article. - Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels.
Nemzou N RM, Bulankina AV, Khimich D, Giese A, Moser T. Nemzou N RM, et al. Neuroscience. 2006 Sep 15;141(4):1849-60. doi: 10.1016/j.neuroscience.2006.05.057. Epub 2006 Jul 10. Neuroscience. 2006. PMID: 16828974 - Myosin VI is required for the proper maturation and function of inner hair cell ribbon synapses.
Roux I, Hosie S, Johnson SL, Bahloul A, Cayet N, Nouaille S, Kros CJ, Petit C, Safieddine S. Roux I, et al. Hum Mol Genet. 2009 Dec 1;18(23):4615-28. doi: 10.1093/hmg/ddp429. Epub 2009 Sep 10. Hum Mol Genet. 2009. PMID: 19744958 - The auditory hair cell ribbon synapse: from assembly to function.
Safieddine S, El-Amraoui A, Petit C. Safieddine S, et al. Annu Rev Neurosci. 2012;35:509-28. doi: 10.1146/annurev-neuro-061010-113705. Annu Rev Neurosci. 2012. PMID: 22715884 Review. - How to build an inner hair cell: challenges for regeneration.
Kros CJ. Kros CJ. Hear Res. 2007 May;227(1-2):3-10. doi: 10.1016/j.heares.2006.12.005. Epub 2006 Dec 16. Hear Res. 2007. PMID: 17258412 Review.
Cited by
- Relating structure and function of inner hair cell ribbon synapses.
Wichmann C, Moser T. Wichmann C, et al. Cell Tissue Res. 2015 Jul;361(1):95-114. doi: 10.1007/s00441-014-2102-7. Epub 2015 Jan 22. Cell Tissue Res. 2015. PMID: 25874597 Free PMC article. Review. - Adenomatous Polyposis Coli Protein Deletion in Efferent Olivocochlear Neurons Perturbs Afferent Synaptic Maturation and Reduces the Dynamic Range of Hearing.
Hickman TT, Liberman MC, Jacob MH. Hickman TT, et al. J Neurosci. 2015 Jun 17;35(24):9236-45. doi: 10.1523/JNEUROSCI.4384-14.2015. J Neurosci. 2015. PMID: 26085645 Free PMC article. - A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function.
Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. Ng L, et al. Endocrinology. 2009 Apr;150(4):1952-60. doi: 10.1210/en.2008-1419. Epub 2008 Dec 18. Endocrinology. 2009. PMID: 19095741 Free PMC article. - Synaptic profiles during neurite extension, refinement and retraction in the developing cochlea.
Huang LC, Barclay M, Lee K, Peter S, Housley GD, Thorne PR, Montgomery JM. Huang LC, et al. Neural Dev. 2012 Dec 7;7:38. doi: 10.1186/1749-8104-7-38. Neural Dev. 2012. PMID: 23217150 Free PMC article.
References
- Barroso I, Benito B, Garci-Jimenez C, Hernandez A, Obregon MJ, Santisteban P. Norepinephrine, tri-iodothyronine and insulin upregulate glyceraldehyde-3-phosphate dehydrogenase mRNA during Brown adipocyte differentiation. Eur J Endocrinol. 1999;141:169–179. - PubMed
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. - PubMed
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous