Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage - PubMed (original) (raw)
Comparative Study
Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage
Ayumu Tashiro et al. J Neurosci. 2007.
Abstract
Neural circuits in the dentate gyrus are continuously modified by adult neurogenesis, whose level is affected by the animal's experience. However, it is not known whether this experience-dependent anatomical modification alters the functional properties of the dentate gyrus. Here, using the expression of immediate early gene products, c-fos and Zif268, as indicators of recently activated neurons, we show that previous exposure to an enriched environment increases the total number of new neurons and the number of new neurons responding to reexposure to the same environment. The increase in the density of activated new neurons occurred specifically in response to exposure to the same environment but not to a different experience. Furthermore, we found that these experience-specific modifications are affected exclusively by previous exposure around the second week after neuronal birth but not later than 3 weeks. Thus, the animal's experience within a critical period during an immature stage of new neurons determines the survival and population response of the new neurons and may affect later neural representation of the experience in the dentate gyrus. This experience-specific functional modification through adult neurogenesis could be a mechanism by which new neurons exert a long-term influence on the function of the dentate gyrus related to learning and memory.
Figures
Figure 1.
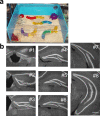
The enriched environment and examined sections from the dentate gyrus. a, A picture of the enriched cage used in this study. b, Representative images of sections used for the analysis from anterior (#1) to posterior (#8). The sections were immunostained with anti-NeuN antibody. Scale bar, 100 μm.
Figure 2.
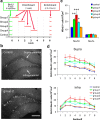
One week preexposure to an enriched environment soon after neuronal birth increases the number of new neurons. a, Timeline of BrdU injections and exposure to an enriched environment. b, Representative images of BrdU-stained dentate sections from the control group and group 2. Scale bar, 100 μm. c, Density of BrdU+/NeuN+ and BrdU+/NeuN− cells. d, Estimated density of BrdU+/NeuN+ cells in the suprapyramidal and infrapyramidal blades of each level along the anteroposterior axis. Section numbers correspond to the numbers in Figure 1_b_. *p < 0.05, **p < 0.005, ***p < 0.0005 by Fisher's LSD test.
Figure 3.
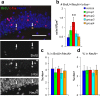
One week preexposure to an enriched environment soon after neuronal birth increases the number of new neurons responding to reexposure to the same environment. a, Confocal images of dentate granule cell layer triple immunostained with BrdU (green), c-fos (red), and NeuN (blue). Arrows indicate triple-positive cells. Scale bar, 50 μm. b, Density of BrdU+/NeuN+/c-fos+ cells in mice exposed to an enriched environment immediately before being perfused. c, Proportions of c-fos+ in BrdU+/NeuN+ cells. d, Proportions of c-fos+ in NeuN+ cells. **p < 0.005 by Fisher's LSD test.
Figure 4.
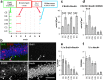
Experience-specific increase in the number of activated new neurons. a, Experimental timeline. b, Confocal images of dentate granule cell layer triple immunostained with BrdU, Zif268, and NeuN. Arrows indicate triple-positive cells. Scale bar, 50 μm. Density of BrdU+/NeuN+ cells (c) and of BrdU+/NeuN+/Zif268+ cells (d) in each group. Proportions of Zif268+ in BrdU+/NeuN+ cells (e) and in NeuN+ cells (f) in each group. **p < 0. 005, ***p < 0.0005 by Fisher's LSD test.
Figure 5.
Long-term survival of mature new neurons does not require continuous exposure to the environment that rescued the new neurons during the critical period. a, Experimental timeline. b, Density of BrdU+/NeuN+ cells in each group.
Comment in
- A critical time for new neurons in the adult hippocampus.
Leuner B, Glasper ER, Mirescu C. Leuner B, et al. J Neurosci. 2007 May 30;27(22):5845-6; discussion 5845. doi: 10.1523/JNEUROSCI.1838-07.2007. J Neurosci. 2007. PMID: 17537953 Free PMC article. Review. No abstract available.
Similar articles
- Active Dentate Granule Cells Encode Experience to Promote the Addition of Adult-Born Hippocampal Neurons.
Kirschen GW, Shen J, Tian M, Schroeder B, Wang J, Man G, Wu S, Ge S. Kirschen GW, et al. J Neurosci. 2017 May 3;37(18):4661-4678. doi: 10.1523/JNEUROSCI.3417-16.2017. Epub 2017 Apr 3. J Neurosci. 2017. PMID: 28373391 Free PMC article. - Transplanted Dentate Progenitor Cells Show Increased Survival in an Enriched Environment But Do Not Exert a Neurotrophic Effect on Spatial Memory Within 2 Weeks of Engraftment.
Jamal AL, Walker TL, Waber Nguyen AJ, Berman RF, Kempermann G, Waldau B. Jamal AL, et al. Cell Transplant. 2015;24(12):2435-48. doi: 10.3727/096368915X687011. Epub 2015 Jan 23. Cell Transplant. 2015. PMID: 25621922 - Strain differences in neurogenesis and activation of new neurons in the dentate gyrus in response to spatial learning.
Epp JR, Scott NA, Galea LA. Epp JR, et al. Neuroscience. 2011 Jan 13;172:342-54. doi: 10.1016/j.neuroscience.2010.10.025. Epub 2010 Oct 16. Neuroscience. 2011. PMID: 20955769 - Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation.
Aasebø IE, Blankvoort S, Tashiro A. Aasebø IE, et al. Eur J Neurosci. 2011 Mar;33(6):1094-100. doi: 10.1111/j.1460-9568.2011.07608.x. Eur J Neurosci. 2011. PMID: 21395853 Review. - Adult neurogenesis and the plasticity of the dentate gyrus network.
Mongiat LA, Schinder AF. Mongiat LA, et al. Eur J Neurosci. 2011 Mar;33(6):1055-61. doi: 10.1111/j.1460-9568.2011.07603.x. Eur J Neurosci. 2011. PMID: 21395848 Review.
Cited by
- Effect of adult hippocampal neurogenesis on pattern separation and its applications.
Wang Z, Yang K, Sun X. Wang Z, et al. Cogn Neurodyn. 2024 Oct;18(5):1-14. doi: 10.1007/s11571-024-10110-3. Epub 2024 Apr 16. Cogn Neurodyn. 2024. PMID: 39568526 - Intracerebral inoculation of healthy non-transgenic rats with a single aliquot of oligomeric amyloid-β (1-42) profoundly and progressively alters brain function throughout life.
Kramer M, Hoang TH, Yang H, Shchyglo O, Böge J, Neubacher U, Colitti-Klausnitzer J, Manahan-Vaughan D. Kramer M, et al. Front Aging Neurosci. 2024 Aug 2;16:1397901. doi: 10.3389/fnagi.2024.1397901. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39156737 Free PMC article. - Adult neurogenesis improves spatial information encoding in the mouse hippocampus.
Frechou MA, Martin SS, McDermott KD, Huaman EA, Gökhan Ş, Tomé WA, Coen-Cagli R, Gonçalves JT. Frechou MA, et al. Nat Commun. 2024 Jul 30;15(1):6410. doi: 10.1038/s41467-024-50699-x. Nat Commun. 2024. PMID: 39080283 Free PMC article. - Modelling adult neurogenesis in the aging rodent hippocampus: a midlife crisis.
Arellano JI, Rakic P. Arellano JI, et al. Front Neurosci. 2024 Jun 3;18:1416460. doi: 10.3389/fnins.2024.1416460. eCollection 2024. Front Neurosci. 2024. PMID: 38887368 Free PMC article. - Tpr Misregulation in Hippocampal Neural Stem Cells in Mouse Models of Alzheimer's Disease.
Malik SC, Lin JD, Ziegler-Waldkirch S, Tholen S, Deshpande SS, Schwabenland M, Schilling O, Vlachos A, Meyer-Luehmann M, Schachtrup C. Malik SC, et al. Cells. 2023 Dec 1;12(23):2757. doi: 10.3390/cells12232757. Cells. 2023. PMID: 38067185 Free PMC article.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. - PubMed
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. - PubMed
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources