Simultaneous induction of the four subunits of the TRAP complex by ER stress accelerates ER degradation - PubMed (original) (raw)
Simultaneous induction of the four subunits of the TRAP complex by ER stress accelerates ER degradation
Koji Nagasawa et al. EMBO Rep. 2007 May.
Abstract
The mammalian translocon-associated protein (TRAP) complex comprises four transmembrane protein subunits in the endoplasmic reticulum. The complex associates with the Sec61 translocon, although its function in vivo remains unknown. Here, we show the involvement of the TRAP complex in endoplasmic reticulum-associated degradation (ERAD). All four subunits are induced simultaneously by endoplasmic reticulum stresses from the X-box-binding protein 1/inositol-requiring 1alpha pathway. RNA interference knockdown of each subunit causes disruption of the native complex and significant delay in the degradation of various ERAD substrates, including the alpha1-antitrypsin null Hong Kong variant (NHK). In a pulse-chase experiment, the TRAP complex associated with NHK at a late stage, indicating its involvement in the ERAD pathway rather than in biosynthesis of nascent polypeptides in the endoplasmic reticulum. In addition, the TRAP complex bound preferentially to misfolded proteins rather than correctly folded wild-type substrates. Thus, the TRAP complex induced by the unfolded protein response pathway might discriminate ERAD substrates from correctly folded substrates, accelerating degradation.
Figures
Figure 1
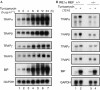
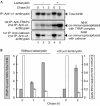
Endoplasmic reticulum stress causes simultaneous induction of each TRAP complex subunit by the X-box-binding protein 1/inositol-requiring 1α pathway. (A) Total RNA was isolated from BALB/c 3T3 cells treated with 5 μg ml−1 tunicamycin for the periods indicated. RNA (5 μg) was analysed by northern hybridization using complementary DNA probes specific to each subunit of mouse TRAP (α, β, γ and δ), mouse BiP (induced by endoplasmic reticulum stress) and human GAPDH (loading control). (B) Wild-type MEF cells (+/+) or IRE1α-knockout MEF cells (−/−) were treated with 5 μg ml−1 tunicamycin for 12 h, followed by northern blot analysis. BiP, immunoglobulin heavy chain binding protein; GAPDH, glyceraldehydes-3-phosphate dehydrogenase; IRE, inositol-requiring; MEF, mouse embryonic fibroblast; TRAP, translocon-associated protein.
Figure 2
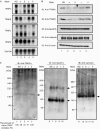
RNA intereference knockdown of each TRAP subunit disrupts the native TRAP complex in the endoplasmic reticulum. Human embryonic kidney 293 cells were transfected with short hairpin RNA expression vectors against each of the TRAP subunits (pSUPER-α1(α), pSUPER-β1(β), pSUPER-γ1(γ) and pSUPER-δ1(δ)) or a nonspecific sequence (pSUPER-NS (NS)). (A) Northern blot analysis of each TRAP subunit after RNAi treatment. Total RNA (10 μg) was used for detection, as described in Fig 1, using probes specific to human TRAP subunits. (B) Western blot analysis of each TRAP subunit after RNAi treatment. Cellular proteins were extracted in NP-40 lysis buffer, and each protein was detected by western blotting. (C) BN–PAGE analysis of the TRAP complex after RNAi treatment. Microsomal fractions (10 or 2 μg protein) were separated by 5–18% BN–PAGE and detected with TRAPα antibody or Sec61β antibody, respectively. Calnexin was used as the loading control (1 μg of sample). The amounts of native TRAP complex remaining after RNAi treatment of each subunit were quantified from three independent analyses and are shown at the bottom of the left panel. The amount of native TRAP complex in the NS lane was set to 100%. BN–PAGE, blue-native–polyacrylamide gel electrophoresis; IB, immunoblotting; RNAi, RNA interferene; TRAP, translocon-associated protein.
Figure 3
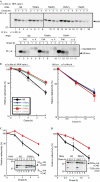
Knockdown of the TRAP complex delays endoplasmic reticulum-associated degradation of misfolded glycoproteins. Human embryonic kidney 293 cells were transiently co-transfected with plasmids encoding endoplasmic reticulum-associated degradation substrates and short hairpin RNA (pSUPER-NS, pSUPER-α1, pSUPER-β1, pSUPER-γ1 or pSUPER-δ1). Degradation of the substrates was examined by metabolic labelling with [35S]Met/Cys for 15 min and by chasing with normal growth medium for the time periods indicated. Intracellular substrates were detected by immunoprecipitation. (A) Degradation of the null Hong Kong (NHK) variant and secretion of wild-type α1-antitrypsin, (C) degradation of the C-terminal-truncated soluble form of ribophorin-1 (RI332) and (D) degradation of the T-cell receptor α-subunit (TCRα) are shown. Quantification analyses of these are shown in (B–D). Radioactivity at chase=0 h was set to 100%, and relative radioactivity compared with this value is shown in the graph. The averages of five (for NHK), four (for wt α1AT) and three (for RI332 and TCRα) independent experiments are plotted and error bars represent s.e.m. CHO refers to _N_-linked glycan. HA, Haemagglutinin; Med, medium; wt α1AT, wild-type α1-antitrypsin.
Figure 4
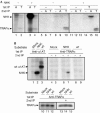
The endogenous TRAP complex preferentially binds to misfolded endoplasmic reticulum-associated degradation substrates. (A) Co-immunoprecipitation (co-IP) of the endogenous TRAP complex with NHK. HEK 293 cells were transfected with NHK (+) or mock plasmid (−), then radiolabelled with [35S]Met/Cys for 2 h, followed by disruption in 3% digitonin lysis buffer. The first IP was carried out with (T) or without (−) TRAPα antibody, and eluates were then subjected to a second IP using α1AT (A), TRAPα antibodies (T) or only protein A beads as a control (−). To detect NHK and the endogenous TRAPα by IP, one-fifth of the volume of the cell lysate used for co-IP was applied to each lane (lanes 1–4). (B) Comparison of the affinity of the TRAP complex for NHK and wild-type α1AT. The second IPs were carried out with (+) or without (−) α1AT antibody (upper panel, lanes 4–9), or with TRAPα antibody (lower panel, lanes 13–15). Control IPs were carried out using α1AT (lanes 1–3) and TRAPα (lanes 10–12) antibodies. α1AT, α1-antitrypsin; HEK, human embryonic kidney; IP, immunopreipitation; Met/Cys, methionine/cysteine; NHK, null Hong Kong variant; TRAP, translocon-associated protein; wt, wild type.
Figure 5
The null Hong Kong variant is co-immunoprecipitated with the TRAP complex during the chase period. (A) Intracellular NHK levels (upper panel) and NHK co-immunoprecipitated with the endogenous TRAP complexes (middle panel) or calnexin (lower panel) in HEK 293 cells transfected with NHK in the presence (+) or absence (−) of lactacystin. The pulse–chase experiment was carried out as in Fig 3, and the time course of NHK degradation and interaction with the TRAP complex was analysed by IP or sequential IP as described in Fig 4A. (B) Quantification of NHK co-IP with TRAP (grey bars) or calnexin (white bars) relative to total NHK. The values at chase=0 h were set as 1 for TRAP-associated (left vertical axis) and calnexin-associated NHK (right vertical axis). The averages of three independent experiments are plotted and error bars indicate s.e.m. (_n_=3). HEK, human embryonic kidney; IP, immunoprecipitation; NHK, null Hong Kong variant; TRAP, translocon-associated protein.
Similar articles
- Protein Translocation Acquires Substrate Selectivity Through ER Stress-Induced Reassembly of Translocon Auxiliary Components.
Lee S, Shin Y, Kim K, Song Y, Kim Y, Kang SW. Lee S, et al. Cells. 2020 Feb 24;9(2):518. doi: 10.3390/cells9020518. Cells. 2020. PMID: 32102453 Free PMC article. - Dissecting the molecular organization of the translocon-associated protein complex.
Pfeffer S, Dudek J, Schaffer M, Ng BG, Albert S, Plitzko JM, Baumeister W, Zimmermann R, Freeze HH, Engel BD, Förster F. Pfeffer S, et al. Nat Commun. 2017 Feb 20;8:14516. doi: 10.1038/ncomms14516. Nat Commun. 2017. PMID: 28218252 Free PMC article. - Translocon-associated protein subunit Trap-γ/Ssr3 is required for vascular network formation in the mouse placenta.
Yamaguchi YL, Tanaka SS, Oshima N, Kiyonari H, Asashima M, Nishinakamura R. Yamaguchi YL, et al. Dev Dyn. 2011 Feb;240(2):394-403. doi: 10.1002/dvdy.22528. Epub 2011 Jan 11. Dev Dyn. 2011. PMID: 21246656 - Understanding the mammalian TRAP complex function(s).
Russo A. Russo A. Open Biol. 2020 May;10(5):190244. doi: 10.1098/rsob.190244. Epub 2020 May 27. Open Biol. 2020. PMID: 32453970 Free PMC article. Review. - The expanding role of the ER translocon in membrane protein folding.
Skach WR. Skach WR. J Cell Biol. 2007 Dec 31;179(7):1333-5. doi: 10.1083/jcb.200711107. J Cell Biol. 2007. PMID: 18166647 Free PMC article. Review.
Cited by
- OST4 is a subunit of the mammalian oligosaccharyltransferase required for efficient N-glycosylation.
Dumax-Vorzet A, Roboti P, High S. Dumax-Vorzet A, et al. J Cell Sci. 2013 Jun 15;126(Pt 12):2595-606. doi: 10.1242/jcs.115410. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606741 Free PMC article. - Structural insights into TRAP association with ribosome-Sec61 complex and translocon inhibition by a CADA derivative.
Pauwels E, Shewakramani NR, De Wijngaert B, Camps A, Provinciael B, Stroobants J, Kalies KU, Hartmann E, Maes P, Vermeire K, Das K. Pauwels E, et al. Sci Adv. 2023 Mar 3;9(9):eadf0797. doi: 10.1126/sciadv.adf0797. Epub 2023 Mar 3. Sci Adv. 2023. PMID: 36867692 Free PMC article. - Exploring the molecular composition of the multipass translocon in its native membrane environment.
Gemmer M, Chaillet ML, Förster F. Gemmer M, et al. Life Sci Alliance. 2024 Jun 12;7(8):e202302496. doi: 10.26508/lsa.202302496. Print 2024 Aug. Life Sci Alliance. 2024. PMID: 38866426 Free PMC article. - Seasonal changes in hepatic gene expression reveal modulation of multiple processes in rainbow smelt (Osmerus mordax).
Richards RC, Short CE, Driedzic WR, Ewart KV. Richards RC, et al. Mar Biotechnol (NY). 2010 Nov;12(6):650-63. doi: 10.1007/s10126-009-9252-8. Epub 2010 Jan 27. Mar Biotechnol (NY). 2010. PMID: 20107851 - NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis.
Gansemer ER, McCommis KS, Martino M, King-McAlpin AQ, Potthoff MJ, Finck BN, Taylor EB, Rutkowski DT. Gansemer ER, et al. iScience. 2020 May 22;23(5):101116. doi: 10.1016/j.isci.2020.101116. Epub 2020 Apr 29. iScience. 2020. PMID: 32417402 Free PMC article.
References
- de Virgilio M, Weninger H, Ivessa NE (1998) Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J Biol Chem 273: 9734–9743 - PubMed
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 - PubMed
- Hartmann E, Gorlich D, Kostka S, Otto A, Kraft R, Knespel S, Burger E, Rapoport TA, Prehn S (1993) A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur J Biochem 214: 375–381 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK042394/DK/NIDDK NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials