Measurement of plasma nitrite by chemiluminescence without interference of S-, N-nitroso and nitrated species - PubMed (original) (raw)
Measurement of plasma nitrite by chemiluminescence without interference of S-, N-nitroso and nitrated species
Enika Nagababu et al. Free Radic Biol Med. 2007.
Abstract
Recent studies have demonstrated that plasma nitrite (NO2-) reflects endothelial nitric oxide synthase activity and it has been proposed as a prognostic marker for cardiovascular disease. In addition, NO2- itself has been shown to have biological activities thought to be triggered by reduction back to NO in blood and tissues. The development of sensitive and reproducible methods for the quantitative determination of plasma NO2- is, therefore, of great importance. Ozone-based chemiluminescence assays have been shown to be highly sensitive for the determination of nanomolar quantities of NO and NO-related species in biological fluids. We report here an improved direct chemiluminescence method for the determination of plasma NO2- without interference of other nitric oxide-related species such as nitrate, S-nitrosothiols, N-nitrosamines, nitrated proteins, and nitrated lipids. The method involves a reaction system consisting of glacial acetic acid and ascorbic acid in the purge vessel of the NO analyzer. Under these acidic conditions NO2- is stoichiometrically reduced to NO by ascorbic acid. Fasting human plasma NO2- values were found in the range of 56-210 nM (mean=110+/-36 nM). This method has high sensitivity with an accuracy of 97% and high precision (CV<10%) for determination of plasma nitrite. The present method is simple and highly specific for plasma NO2-. It is particularly suited for evaluating vasculature endothelial NO production that predicts the risks for cardiovascular disease.
Figures
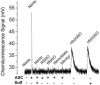
Fig.1
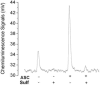
A) Chemiluminescence signals obtained under acidic conditions. The purge vessel contained 87.5% glacial acetic acid in absence and presence of 50 mM ascorbic acid and/or 200mM sulfanilamide in a total volume of 8 ml. Samples injected into the purge vessel were: 20 pmoles nitrite, 100 pmoles of each RSNO (GSNO or albumin-SNO), RNNO (_N_-nitrosodimethylamine or _N_-nitroso-N-methylurea), nitrooleate, nitrotyr (nitrotyrosine) or Hb(II)NO (iron nitrosylhemoglobin). ASC indicates the addition of ascorbic acid; Sulf indicates the addition of sulfanilamide. B) Release of NO from S- nitrosoalbumin by KI or I3- or ascorbate in glacial acetic acid. The reaction mixture for I3- chemiluminescence assay consisted of 7 ml glacial acetic acid, 0.5 ml (1M) KI solution and 0.5 ml (0.35 M) I2 solution. The reaction mixture for KI assay consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) KI solution. The reaction mixture for ascorbate consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbate solution. 50 pmoles S-nitrosoalbumin was injected into the purge vessel.
Fig.1
A) Chemiluminescence signals obtained under acidic conditions. The purge vessel contained 87.5% glacial acetic acid in absence and presence of 50 mM ascorbic acid and/or 200mM sulfanilamide in a total volume of 8 ml. Samples injected into the purge vessel were: 20 pmoles nitrite, 100 pmoles of each RSNO (GSNO or albumin-SNO), RNNO (_N_-nitrosodimethylamine or _N_-nitroso-N-methylurea), nitrooleate, nitrotyr (nitrotyrosine) or Hb(II)NO (iron nitrosylhemoglobin). ASC indicates the addition of ascorbic acid; Sulf indicates the addition of sulfanilamide. B) Release of NO from S- nitrosoalbumin by KI or I3- or ascorbate in glacial acetic acid. The reaction mixture for I3- chemiluminescence assay consisted of 7 ml glacial acetic acid, 0.5 ml (1M) KI solution and 0.5 ml (0.35 M) I2 solution. The reaction mixture for KI assay consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) KI solution. The reaction mixture for ascorbate consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbate solution. 50 pmoles S-nitrosoalbumin was injected into the purge vessel.
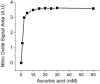
Fig. 2
Ascorbate concentration dependent nitrite reduction to NO under acidic conditions. The reaction mixture contained 7 ml of glacial acetic acid and varying concentration of ascorbic acid in a total volume of 8 ml. 25 pmoles nitrite was injected into the purge vessel. A.U, Arbitrary Units.
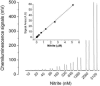
Fig.3
Chemiluminescence signals of nitrite and standard curve. (A) 100 μl of 10 nM to 5120 nM nitrite was injected into reaction mixture contained 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbic acid solution (inset shows standard curve obtained by integrating the area under the curve using the Origin program) (B) Standard curve for nitrite from 10nM to 640 nM. The values are the mean of 3 experiments with duplicate determinations for each experiment.
Fig.3
Chemiluminescence signals of nitrite and standard curve. (A) 100 μl of 10 nM to 5120 nM nitrite was injected into reaction mixture contained 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbic acid solution (inset shows standard curve obtained by integrating the area under the curve using the Origin program) (B) Standard curve for nitrite from 10nM to 640 nM. The values are the mean of 3 experiments with duplicate determinations for each experiment.
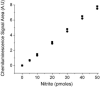
Fig.4
The reduction of nitrite to NO by the ASC reductive chemiluminescence assay is comparable with I3- reductive chemiluminescence assay. The reaction mixture for I3- (square) and ASC chemiluminescence assay (circle) was as mentioned in figure 1B. 10 μl to 250 μl of 0.2 μM nitrite that corresponds to 10 pmoles, 20 pmoles, 30 pmoles, 40 pmoles and 50 pmoles were injected into the purge vessel. Values are the mean of 3 experiments.
Fig.5
Chemiluminescence signals of plasma nitrite: Peak 1, 100 μl plasma was injected into 8 ml of 87.5% glacial acetic acid reagent. This peak was completely suppressed in the presence of 200mM sulfanilamide (sulfa) in 87.5% glacial acetic acid. Peak 2, 100 μl plasma was injected into7 ml glacial acetic acid and 1 ml (0.5M) ascorbic acid. This peak was almost completely suppressed in the presence of 200 mM sulfa in 87.5% glacial acetic acid and 1 ml (0.5M) ascorbic acid.
Fig.6
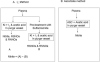
Flow diagram for determination of plasma nitrite using (A) I3- reductive chemiluminescence method and (B) ascorbate reductive chemiluminescence method.
Fig.7
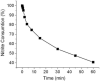
Nitrite consumption in blood: Nitrite (1μM) was added to venous blood and kept on ice. The first aliquot was centrifuged within 10 sec to separate plasma. This plasma nitrite value was considered as 100%. Additional aliquots were centrifuged at different times followed by a determination of the nitrite in the supernatant.
Fig.8
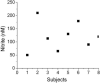
Nitrite levels in human plasma: 100μl plasma was injected into the purge vessel that contained 7 ml of glacial acetic acid and 1 ml of 0.5 M ascorbic acid at 37 °C. Values are the mean of quadruplicates for each sample.
Similar articles
- Measurement of plasma nitrite by chemiluminescence.
Nagababu E, Rifkind JM. Nagababu E, et al. Methods Mol Biol. 2010;610:41-9. doi: 10.1007/978-1-60327-029-8_3. Methods Mol Biol. 2010. PMID: 20013171 Free PMC article. - Optimization of nitric oxide chemiluminescence operating conditions for measurement of plasma nitrite and nitrate.
Bateman RM, Ellis CG, Freeman DJ. Bateman RM, et al. Clin Chem. 2002 Mar;48(3):570-3. Clin Chem. 2002. PMID: 11861453 No abstract available. - Specific S-nitrosothiol (thionitrite) quantification as solution nitrite after vanadium(III) reduction and ozone-chemiluminescent detection.
Ewing JF, Janero DR. Ewing JF, et al. Free Radic Biol Med. 1998 Sep;25(4-5):621-8. doi: 10.1016/s0891-5849(98)00083-5. Free Radic Biol Med. 1998. PMID: 9741600 - Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids.
Tsikas D. Tsikas D. Free Radic Res. 2005 Aug;39(8):797-815. doi: 10.1080/10715760500053651. Free Radic Res. 2005. PMID: 16036360 Review. - Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research.
Tsikas D. Tsikas D. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 May 15;851(1-2):51-70. doi: 10.1016/j.jchromb.2006.07.054. Epub 2006 Sep 6. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 16950667 Review.
Cited by
- Stability and Reproducibility of the Measurement of Plasma Nitrate in Large Epidemiologic Studies.
Wang Y, Townsend MK, Eliassen AH, Wu T. Wang Y, et al. N Am J Med Sci (Boston). 2013 Apr 1;6(2):82-86. N Am J Med Sci (Boston). 2013. PMID: 24244804 Free PMC article. - Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation.
Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM. Cao Z, et al. Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1494-503. doi: 10.1152/ajpheart.01233.2008. Epub 2009 Aug 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19700624 Free PMC article. - The quaternary hemoglobin conformation regulates the formation of the nitrite-induced bioactive intermediate and the dissociation of nitric oxide from this intermediate.
Rifkind JM, Nagababu E, Ramasamy S. Rifkind JM, et al. Nitric Oxide. 2011 Mar 15;24(2):102-9. doi: 10.1016/j.niox.2011.01.001. Epub 2011 Jan 12. Nitric Oxide. 2011. PMID: 21236353 Free PMC article. - Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man.
Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, Söderberg S, Pourazar J, Megson IL, Treweeke A, Sandström T, Newby DE, Blomberg A, Mills NL. Langrish JP, et al. J Am Heart Assoc. 2013 Feb 19;2(1):e004309. doi: 10.1161/JAHA.112.004309. J Am Heart Assoc. 2013. PMID: 23525434 Free PMC article. Clinical Trial. - The Generation of Nitric Oxide from Aldehyde Dehydrogenase-2: The Role of Dietary Nitrates and Their Implication in Cardiovascular Disease Management.
Maiuolo J, Oppedisano F, Carresi C, Gliozzi M, Musolino V, Macrì R, Scarano F, Coppoletta A, Cardamone A, Bosco F, Mollace R, Muscoli C, Palma E, Mollace V. Maiuolo J, et al. Int J Mol Sci. 2022 Dec 7;23(24):15454. doi: 10.3390/ijms232415454. Int J Mol Sci. 2022. PMID: 36555095 Free PMC article. Review.
References
- Marin J, Rodriguez-Martinez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther. 1997;75:111–134. - PubMed
- Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur Heart J. 1997;18(Suppl E):E19–29. - PubMed
- Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. - PubMed
- Babaei S, Stewart DJ. Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc Res. 2002;55:190–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources