Transcriptional regulation of T cell tolerance - PubMed (original) (raw)
Review
Transcriptional regulation of T cell tolerance
Sanmay Bandyopadhyay et al. Semin Immunol. 2007 Jun.
Abstract
Self-reactive T cells that escape negative selection in the thymus must be kept under control in the periphery. Mechanisms of peripheral tolerance include deletion or functional inactivation of self-reactive T cells and mechanisms of dominant tolerance mediated by regulatory T cells. In the absence of costimulation, T cell receptor (TCR) engagement results in unopposed calcium signaling that leads to the activation of a cell-intrinsic program of inactivation, which makes T cells hyporesponsive to subsequent stimulations. The activation of this program in anergic T cells is a consequence of the induction of a nuclear factor of activated T cells (NFAT)-dependent program of gene expression. Recent studies have offered new insights into the mechanisms responsible for the implementation and maintenance of T cell anergy and have provided evidence that the proteins encoded by the genes upregulated in anergic T cells are responsible for the implementation of anergy by interfering with TCR signaling or directly inhibiting cytokine gene transcription.
Figures
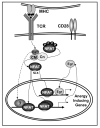
Figure 1. NFAT induces a specific program of gene expression in anergic T cells
TCR engagement without concomitant costimulation results in the unbalance activation of calcium signaling in the absence of full activation of other pathways (i.e. Ras/MAPK, PKC, IKK). Sustained increased intracellular calcium activates the calmodulin (CM) dependent phosphatase calcineurin (Cn) which dephosphorylates NFAT, exposing a nuclear localization signal (NLS) and inducing NFAT nuclear translocation. In the absence of AP-1 proteins, NFAT induces an anergy-inducing program of gene expression. NFAT also upregulates the expression of Egr2 and Egr3 (Egr) that contribute in cooperation with NFAT or in an independent manner, to activate the expression of anergy-inducing genes. The proteins encoded by those genes (e.g. Itch, Grail, Cbl-b, DGKα, Ikaros) will cause dampening of TCR signaling and IL-2 silencing in anergic T cells.
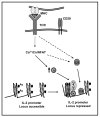
Figure 2. Active mechanisms of transcriptional repression contribute to the implementation and maintenance of anergy in T cells
Anergizing stimuli induce the calcium/NFAT-dependent upregulation of the expression of Ikaros (Ik). In anergic T cells Ikaros binds to the IL-2 promoter and recruits histone deacetylases (HDAC). These enzymes would remove acetyl groups (Ac) from core nucleosome histones (H) leading to the establishment of epigenetic changes that result in stable silencing of the IL-2 gene expression. Other transcriptional repressors (i.e. CREM proteins and Smad3 (C/S)) may be activated by calcium or other signals and bind to the IL-2 promoter also inhibiting IL-2 expression.
Similar articles
- IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes.
Duré M, Macian F. Duré M, et al. Mol Immunol. 2009 Feb;46(5):999-1006. doi: 10.1016/j.molimm.2008.09.029. Epub 2008 Nov 5. Mol Immunol. 2009. PMID: 18990450 Free PMC article. - E3 ligases in T cell anergy--turning immune responses into tolerance.
Heissmeyer V, Rao A. Heissmeyer V, et al. Sci STKE. 2004 Jul 6;2004(241):pe29. doi: 10.1126/stke.2412004pe29. Sci STKE. 2004. PMID: 15252218 Review. - Regulation of T-cell tolerance by calcium/NFAT signaling.
Baine I, Abe BT, Macian F. Baine I, et al. Immunol Rev. 2009 Sep;231(1):225-40. doi: 10.1111/j.1600-065X.2009.00817.x. Immunol Rev. 2009. PMID: 19754900 Review. - T-cell anergy.
Macián F, Im SH, García-Cózar FJ, Rao A. Macián F, et al. Curr Opin Immunol. 2004 Apr;16(2):209-16. doi: 10.1016/j.coi.2004.01.013. Curr Opin Immunol. 2004. PMID: 15023415 Review. - Transcriptional basis of lymphocyte tolerance.
Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Borde M, et al. Immunol Rev. 2006 Apr;210:105-19. doi: 10.1111/j.0105-2896.2006.00370.x. Immunol Rev. 2006. PMID: 16623767 Review.
Cited by
- FoxO-dependent regulation of diacylglycerol kinase α gene expression.
Martínez-Moreno M, García-Liévana J, Soutar D, Torres-Ayuso P, Andrada E, Zhong XP, Koretzky GA, Mérida I, Ávila-Flores A. Martínez-Moreno M, et al. Mol Cell Biol. 2012 Oct;32(20):4168-80. doi: 10.1128/MCB.00654-12. Epub 2012 Aug 13. Mol Cell Biol. 2012. PMID: 22890845 Free PMC article. - Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response.
Reiser J, Banerjee A. Reiser J, et al. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. Epub 2016 May 22. J Immunol Res. 2016. PMID: 27314056 Free PMC article. Review. - Silencing of the Il2 gene transcription is regulated by epigenetic changes in anergic T cells.
Bandyopadhyay S, Montagna C, Macian F. Bandyopadhyay S, et al. Eur J Immunol. 2012 Sep;42(9):2471-83. doi: 10.1002/eji.201142307. Epub 2012 Jul 13. Eur J Immunol. 2012. PMID: 22684523 Free PMC article. - Protein ubiquitination in T cell development.
Zhong T, Lei K, Lin X, Xie Z, Luo S, Zhou Z, Zhao B, Li X. Zhong T, et al. Front Immunol. 2022 Aug 4;13:941962. doi: 10.3389/fimmu.2022.941962. eCollection 2022. Front Immunol. 2022. PMID: 35990660 Free PMC article. Review. - IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes.
Duré M, Macian F. Duré M, et al. Mol Immunol. 2009 Feb;46(5):999-1006. doi: 10.1016/j.molimm.2008.09.029. Epub 2008 Nov 5. Mol Immunol. 2009. PMID: 18990450 Free PMC article.
References
- Macian F, Im SH, Garcia-Cozar FJ, Rao A. T-cell anergy. Curr Opin Immunol. 2004;16:209–16. - PubMed
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. - PubMed
- Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–9. - PubMed
- Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI059738-03/AI/NIAID NIH HHS/United States
- R56 AI059738/AI/NIAID NIH HHS/United States
- GM007288/GM/NIGMS NIH HHS/United States
- T32 GM007491-27/GM/NIGMS NIH HHS/United States
- AI059738/AI/NIAID NIH HHS/United States
- T32 GM007491/GM/NIGMS NIH HHS/United States
- R01 AI059738/AI/NIAID NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources