Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing - PubMed (original) (raw)
Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing
Susan Roehl White et al. J Cell Biol. 2007.
Abstract
Spastin, an AAA ATPase mutated in the neurodegenerative disease hereditary spastic paraplegia, severs microtubules. Many other AAA proteins form ring-shaped hexamers and contain pore loops, which project into the ring's central cavity and act as ratchets that pull on target proteins, leading, in some cases, to conformational changes. We show that Spastin assembles into a hexamer and that loops within the central pore recognize C-terminal amino acids of tubulin. Key pore loop amino acids are required for severing, including one altered by a disease-associated mutation. We also show that Spastin contains a second microtubule binding domain that makes a distinct interaction with microtubules and is required for severing. Given that Spastin engages the MT in two places and that both interactions are required for severing, we propose that severing occurs by forces exerted on the C-terminal tail of tubulin, which results in a conformational change in tubulin, which releases it from the polymer.
Figures
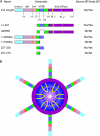
Figure 1.
Schematic depiction of domain architecture of Spastin and constructs used in this study. (A) The numbers below each box represent the amino acid boundaries of individual domains/deletions. Numbering based on human Spastin. PL, pore loop. The column on the right indicates whether the mutant severs and/or binds to MTs. (B) Model of hexameric Spastin showing central pore and location of pore loops and N-terminal MTBD. The majority of the ring is formed by the AAA ATPase domain (purple). Image is looking down into the pore cavity. Each monomer of Spastin contributes two pore loops. Pore loop 1 (yellow) lies near the mouth of the pore, whereas pore loop 2 (pink) resides deeper in the pore. The N-terminal region projects from the ring. The cartoon is loosely based on structures of many AAA ATPases.
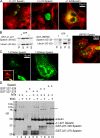
Figure 2.
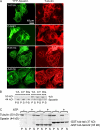
Identification of an N-terminal MTBD in Spastin. (A) Cos-7 cells transfected with plasmids encoding YFP-Spastin (green) with the indicated amino acids deleted were fixed and stained for tubulin (red). Deletion of the first 227 or even the first 279 amino acids did not abolish severing, as the transfected cell lacked MTs (compared with surrounding nontransfected cells). In contrast, deletion of the first 328 amino acids did abolish severing activity, as the transfected cell still has an MT array. This suggested that the approximate boundaries for a MTBD are between aa 280 and 328. (B) Recombinant Δ1–227 binds to taxol-stabilized MTs in the absence of ATP and severs them in the presence of ATP. Δ1–227 Spastin was incubated with taxol-stabilized MTs either in the absence or presence of ATP as indicated. MTs were recovered in the pellet (P) fractions after centrifugation. In the absence of ATP, some of the Δ–1227 Spastin bound to MTs. In the presence of ATP, most of the tubulin was recovered in the supernatant fraction, indicative of MT disassembly. Most of the Spastin was recovered in the supernatant as well, except for a small fraction that sedimented nonspecifically. In contrast, ΔMTBD Spastin neither binds nor severs MTs, even in the presence of ATP. ΔMTBD Spastin was incubated with ATP, as indicated, in either the absence (left two lanes) or presence of taxol-stabilized MTs (right two lanes). Samples were centrifuged, and pellet and supernatant fractions were examined by SDS-PAGE. A small percentage of the mutant Spastin sedimented nonspecifically in the absence of MTs. Note that there was no specific binding of the mutant Spastin to MTs (no additional Spastin in the pellet fraction with the MTs) and that the vast majority of the tubulin was recovered in the pellet, indicating that severing did not occur. In the gel on the right, samples were all from the same experiment but were run on opposite ends of a single gel and so are shown separately here. The micrograph shows that cells transfected with a plasmid encoding YFP-ΔMTBD Spastin still have MT, arrays showing that the enzyme does not sever in cells (compare to Δ–1227 Spastin in Fig. 2 A). (C) To determine whether the MTBD is sufficient to confer MT association, stop codons were introduced at the indicated positions in the full-length YFP-Spastin cDNA, and the indicated plasmids were used to transfect cells. 1–279 stop Spastin did not decorate MTs (inset shows MTs). In contrast, 1–328 stop Spastin decorated MTs, suggesting that a region between 280 and 328 may be sufficient for MT binding. (D) Chemical cross-linking demonstrates an interaction between the MTBD and MTs. Taxol-stabilized MTs were incubated with Spastin, GST-227-279 Spastin, or GST-227-328 Spastin, as indicated in the figure, in the presence of EDC as indicated. The Coomassie blue–stained gel shows that neither GST fusion protein cross-linked to itself in the absence of MTs (lanes 3 and 4) but that GST-227-328 Spastin cross-linked nearly as efficiently to MTs (lane 8; cross-link marked by two asterisks), as did Spastin (lane 10; cross-link marked by three asterisks). The cross-link marked by a single asterisk is a tubulin–tubulin cross-link (see Fig. S2, available at
http://www.jcb.org/cgi/content/full/jcb.200610072/DC1
).
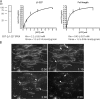
Figure 3.
Biochemical characterization of Δ1–227 Spastin. (A) An aliquot of purified Δ1–227 Spastin was analyzed by SDS-PAGE. ATPase activity as a function of ATP concentration is shown. The data was fit to a curve using Prism 4.0 software, and nonlinear regression analysis was used to calculate Km and Vmax. Km and Vmax were also recalculated from our original data (Evans et al., 2005) for full-length Spastin using nonlinear regression method rather than the Lineweaver-Burke plot originally used. Error bars indicate ± SD. (B) Spastin Δ1–227 severs MTs. Rhodamine-labeled taxol-stabilized MTs (1 μM tubulin) were immobilized in a perfusion chamber, and 400 nM Spastin with 1 mM ATP was added. Four frames from the time lapse are shown with the time stamped (min:s) in each frame. Note the appearance of internal breaks indicative of severing. Representative breaks are indicated by arrowheads. Conditions are as published previously (Evans et al., 2005).
Figure 4.
Spastin binds the C-terminal tail of tubulin and hexamerizes in an ATP-dependent manner. GST–tubulin tails (ending in either Tyr or Glu) were immobilized on glutathione–Sepharose. WT or E442Q Spastin was applied in either the absence or presence of ATP. After binding and washing of beads, samples were analyzed by SDS-PAGE. (A) E442Q but not WT Spastin binds in the presence of ATP. The first lane of each series shows an aliquot (20% of total) of Spastin starting material. About 25–33% of the Spastin bound to the tails. (B) Compared with full-length tail, deletion of the last 22 amino acids from the tail nearly completely abrogates the binding of E442Q Spastin. (C) Purified Δ1–227 Spastin was subjected to gel filtration chromatography on a Superdex 200 column. 0.4-ml fractions were analyzed by SDS-PAGE and Coomassie blue staining. Elution positions of size standards are shown. Elution times in minutes are shown below each fraction. (D) Sedimentation velocity analysis of E442Q Spastin in the presence of ATP. Normalized g(s*) versus s* plots were obtained using the program DcDt+ (Philo, 2000, 2006). Sample concentrations: 0.94 mg/ml (black), 0.51 mg/ml (blue), 0.22 mg/ml (red), and 0.08 mg/ml (green).
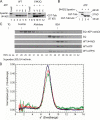
Figure 5.
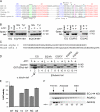
Mutations in pore loops 1 and 2 affect tubulin tail recognition. (A) Sequence alignments of part of the AAA domain from several AAA proteins. Alignments are from Karata et al. (1999). Pore loop 1 is underlined and contains the YVG motif (green). This loop, which projects into the pore near the “mouth,” lies between sheet β2 and helix α3 in AAA domains. Pore loop 2 projects into the pore cavity but lies deeper in the pore and follows the Walker B motif (DEVD in Spastin) and lies between this motif and helix α4 in the AAA domain. Amino acids mutated in pore loop 2 are marked by asterisks. (B) Effect of pore loop mutations on α-tubulin C-terminal tail binding. (left) Although the pore loop 1 mutation (Y415A) did not bind the α-tubulin tail, the HSP-associated pore loop 2 mutation (C448Y) did not affect tail binding. (right) Either elimination of a basic residue (R451G) or introduction of an acidic residue (A457E) in pore loop 2 abolishes tail binding to both Glu (E) and Tyr (Y) tails. The tail binding assay is as described in Fig. 4. In all cases, the indicated pore loop mutations were engineered into E442Q Spastin and we were asking whether the pore loop mutations abolished binding of hexamerized Spastin. (C) Partial amino acid sequences of mouse α2- and β3-tubulin tails are shown. Note the differences at extreme C terminus. (D) Binding of E442Q Spastin to the β3-tubulin tail and the effects of pore loop mutations. Samples marked L represent 20% of the input Spastin. As a positive control, lane 2 shows binding to the α2-tubulin tail. Lanes 3 and 4 show that E442Q Spastin binds to the β3-tubulin tail in an ATP-dependent manner. As with the α tail, the YA, RG, and AE mutants do not bind. Although C448Y binds the α2 tail, it does not bind to the β3 tail. (E) Relative ATPase activities of Spastin proteins harboring pore loop mutants. Error bars indicate ± SD. (F) Selected pore loop mutants that are impaired in tubulin tail binding are not impaired in oligomer formation. Gel filtration analysis performed in the presence of ATP with the indicated mutant Spastin proteins shows that homooligomerization is not abolished by the pore loop mutations. Because we only detect stable oligomer formation with E442Q Spastin, we introduced the R451G or A457E mutations into E442Q Spastin. Note that both mutant proteins assemble (peak eluting around minutes 28–30). The elution positions of size standards are shown.
Figure 6.
Pore loop mutations affect MT-severing activity. (A) Pore loop mutations abolish MT severing in vivo. Although overexpression of YFP-Spastin results in MT loss (Fig. 2 A), overexpression of Spastin harboring pore loop mutations (green) did not (tubulin shown in red). Instead, these mutant proteins decorate a subset of MTs, as do many other Spastin mutants (Evans et al., 2005). (B) Pore loop mutations abolish MT severing in vitro. The indicated mutant Spastin proteins were incubated with ATP in either the absence or presence of taxol-stabilized MTs. After incubation, samples were centrifuged. In contrast to WT Spastin, which releases severed tubulin that is recovered in the supernatant (S) fractions after centrifugation (Fig. 2 B), these mutants do not sever. MTs are in the pellet (P) fractions. Mutant proteins did not sediment in the absence of MTs. A fraction of each protein sedimented with the MTs. (C) Peptides corresponding to the C-terminal tail of tubulin are competitive inhibitors of MT severing. Taxol-stabilized MTs were incubated with Spastin and ATP as indicated. Where indicated, purified GST–tubulin tail (GST fused to the C-terminal 104 amino acids of α-tubulin) or GST–tubulin tail lacking the C-terminal 22 amino acids was added as a competitive inhibitor at 10-fold molar excess compared with tubulin (threefold molar excess compared with Spastin hexamer assuming all Spastin oligomerizes). After 15 min at 37°C, MTs were separated from released tubulin by centrifugation as described previously (Evans et al., 2005). Severing activity is evident by appearance of more tubulin in supernatant fractions. Remaining MTs are recovered in the pellet.
Figure 7.
Spastin interacts only poorly with nonpolymerized tubulin. (A) GST-E442Q Spastin was incubated with ATP and immobilized on glutathione–Sepharose. 12 μg tubulin was added to 6 μl bead slurry containing 12 μg Spastin in a volume of 100 μl. After incubation for 30 min at room temperature, beads were washed three times. Almost none of the tubulin bound to the immobilized Spastin (lane 2). Lane 1 shows 20% of the tubulin load. These two lanes are from the same experiment but were run on opposite ends of the same gel and so are shown separately here. (B) Equivalent amounts of nonpolymerized tubulin (lanes 1–4) or taxol-stabilized MTs (lanes 5–8) were incubated with E442Q Spastin, ATP, and EDC, as indicated in the figure. The Coomassie blue–stained gel shows that Spastin only cross-links weakly to nonpolymerized tubulin (lane 4) compared with MT polymer (lane 8). The cross-link marked by the asterisk is uncharacterized (see Fig. S1, available at
http://www.jcb.org/cgi/content/full/jcb.200610072/DC1
).
Figure 8.
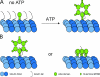
Model. (A) Unassembled Spastin can associate with MT polymer by virtue of the N-terminal MTBD. This interaction is not ATP dependent. ATP results in assembly of the hexamer and pore formation. The tubulin tails, which project from the surface of the MT polymer, bind to the pore loops (tails not drawn on all tubulins for clarity). This binding is ATP dependent. Thus, Spastin can engage the MT in two distinct places. (B) The orientation of the ring on MTs is unclear. Two possible orientations are shown. The number of N-terminal MTBDs simultaneously in contact with the MT is not known.
Similar articles
- Conserved aromatic and basic amino acid residues in the pore region of Caenorhabditis elegans spastin play critical roles in microtubule severing.
Matsushita-Ishiodori Y, Yamanaka K, Hashimoto H, Esaki M, Ogura T. Matsushita-Ishiodori Y, et al. Genes Cells. 2009 Aug;14(8):925-40. doi: 10.1111/j.1365-2443.2009.01320.x. Epub 2009 Jul 13. Genes Cells. 2009. PMID: 19619244 - Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin.
Roll-Mecak A, Vale RD. Roll-Mecak A, et al. Nature. 2008 Jan 17;451(7176):363-7. doi: 10.1038/nature06482. Nature. 2008. PMID: 18202664 Free PMC article. - Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing.
Evans KJ, Gomes ER, Reisenweber SM, Gundersen GG, Lauring BP. Evans KJ, et al. J Cell Biol. 2005 Feb 14;168(4):599-606. doi: 10.1083/jcb.200409058. J Cell Biol. 2005. PMID: 15716377 Free PMC article. - The AAA ATPase spastin links microtubule severing to membrane modelling.
Lumb JH, Connell JW, Allison R, Reid E. Lumb JH, et al. Biochim Biophys Acta. 2012 Jan;1823(1):192-7. doi: 10.1016/j.bbamcr.2011.08.010. Epub 2011 Aug 25. Biochim Biophys Acta. 2012. PMID: 21888932 Review. - Hereditary spastic paraplegia SPG4: what is known and not known about the disease.
Solowska JM, Baas PW. Solowska JM, et al. Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review.
Cited by
- Spastin interacts with collapsin response mediator protein 3 to regulate neurite growth and branching.
Ji ZS, Li JP, Fu CH, Luo JX, Yang H, Zhang GW, Wu W, Lin HS. Ji ZS, et al. Neural Regen Res. 2021 Dec;16(12):2549-2556. doi: 10.4103/1673-5374.313052. Neural Regen Res. 2021. PMID: 33907047 Free PMC article. - Binding of Substrates to the Central Pore of the Vps4 ATPase Is Autoinhibited by the Microtubule Interacting and Trafficking (MIT) Domain and Activated by MIT Interacting Motifs (MIMs).
Han H, Monroe N, Votteler J, Shakya B, Sundquist WI, Hill CP. Han H, et al. J Biol Chem. 2015 May 22;290(21):13490-9. doi: 10.1074/jbc.M115.642355. Epub 2015 Apr 1. J Biol Chem. 2015. PMID: 25833946 Free PMC article. - Spastin regulates anaphase chromosome separation distance and microtubule-containing nuclear tunnels.
Kelley ME, Carlini L, Kornakov N, Aher A, Khodjakov A, Kapoor TM. Kelley ME, et al. Mol Biol Cell. 2024 Apr 1;35(4):ar48. doi: 10.1091/mbc.E24-01-0031-T. Epub 2024 Feb 9. Mol Biol Cell. 2024. PMID: 38335450 Free PMC article. - Spastin Promotes the Migration and Invasion Capability of T98G Glioblastoma Cells by Interacting with Pin1 through Its Microtubule-Binding Domain.
Temizci B, Kucukvardar S, Karabay A. Temizci B, et al. Cells. 2023 Jan 27;12(3):427. doi: 10.3390/cells12030427. Cells. 2023. PMID: 36766769 Free PMC article. - Katanin regulates dynamics of microtubules and biogenesis of motile cilia.
Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, Gaertig J. Sharma N, et al. J Cell Biol. 2007 Sep 10;178(6):1065-79. doi: 10.1083/jcb.200704021. J Cell Biol. 2007. PMID: 17846175 Free PMC article.
References
- Baas, P.W., A. Karabay, and L. Qiang. 2005. Microtubules cut and run. Trends Cell Biol. 15:518–524. - PubMed
- Cassimeris, L. 2002. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 14:18–24. - PubMed
- Chapin, S.J., and J.C. Bulinski. 1991. Preparation and functional assay of pure populations of tyrosinated and detyrosinated tubulin. Methods Enzymol. 196:254–264. - PubMed
- Charvin, D., C. Cifuentes-Diaz, N. Fonknechten, V. Joshi, J. Hazan, J. Melki, and S. Betuing. 2003. Mutations of SPG4 are responsible for a loss of function of spastin, an abundant neuronal protein localized in the nucleus. Hum. Mol. Genet. 12:71–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 NS046472/NS/NINDS NIH HHS/United States
- R01 NS043298/NS/NINDS NIH HHS/United States
- T32 MH015174/MH/NIMH NIH HHS/United States
- T32 MH15174-28/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases