Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons - PubMed (original) (raw)
Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons
Xiang Zhou et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The molecular mechanism and significance of endocytic processes involved in directional axon elongation are not well understood. The Unc-51 family of serine/threonine kinases was shown to be important for axon growth and was also linked to endocytosis, providing an entry point to study this problem. We found that mouse Unc-51-like kinase 1/2 (Ulk1/2) proteins are localized to vesicular structures in growth cones of mouse spinal sensory neurons. RNAi-mediated knockdown of Ulk1 and/or Ulk2 resulted in impaired endocytosis of nerve growth factor (NGF), excessive axon arborization, and severely stunted axon elongation. The evidence also indicates that Ulk1/2 mediates a non-clathrin-coated endocytosis in sensory growth cones. Interestingly, NGF can induce the interaction of Ulk1 with TrkA receptor complexes through promoting K63-polyubiquitination of Ulk1 and binding of Ulk1 to the scaffolding protein p62. These results and additional studies suggest that Ulk1/2 proteins regulate filopodia extension and neurite branching during sensory axon outgrowth, probably through regulating TrkA receptor trafficking and signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
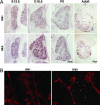
Expression and localization of Ulk1 and Ulk2 in mouse sensory neurons. (A) In situ hybridization experiments show the expression of Ulk1 (Upper) and Ulk2 (Lower) genes in mouse DRG neurons at four different stages: E12.5, E16.5, postpartum day 0 (P0), and adult. (B) Antibody staining reveals that Ulk1 and Ulk2 are present in sensory axons and growth cones and often localize to punctuated structures (presumably vesicles).
Fig. 2.
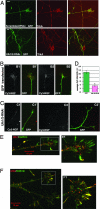
RNAi-mediated knockdown of Ulk1/2 caused exuberant axon branching in embryonic sensory neurons. Different pSUPER-based constructs were cotransfected with EGFP into E12.5 DRG neurons and cultured for 60 h. Representative images are shown here. All pictures are taken with the same magnification. (Scale bar: 100 μm.) (A) A neuron transfected with GFP alone shows bipolar morphology with two main long axons and two terminal branches. (B) A neuron transfected with scrambled-RNAi plus GFP plasmid shows bipolar morphology with two long axons and limited terminal branching. (C) Three Ulk1-RNAi plus GFP-transfected neurons all have multiple branches and shorter axons. (D) Two Ulk2-RNAi plus GFP-transfected neurons grow multiple branches. (E) Three neurons transfected with both Ulk1-RNAi and Ulk2-RNAi, as well as GFP, all have complex axon arborizations. (F) Percentage of neurons with different numbers of total axon branches. In each histogram, the y axis is the percentage of neurons; the x axis is the number of total branches grouped into five columns: 1–4, 5–7, 8–12, 13–22, and 23–30.
Fig. 3.
Ulk1/2 may mediate a non-clathrin-coated vesicle endocytosis to regulate NGF internalization. (A) DRG were transfected with constructs as indicated on the figures and cultured for 60 h. Cells were fixed and stained with anti-TrkA under nonpermeabilizing (surface TrkA) conditions. TrkA was equally expressed on the surface of axons and growth cones in both control and Ulk1/2-RNAi-transfected neurons and on the numerous arbors of the Ulk1/2-RNAi-expressing neurons. (Scale bar: 10 μm.) (B) Representative images of Cy3-NGF internalization into the growth cones of GFP-expressing neurons. B1 and B2 show Cy3-images alone; B1′ and B2′ are merged images of Cy3 and GFP. (C) Representative images of Cy3-NGF internalization into the growth cones of Ulk1/2-RNAi-expressing neurons. C1 and C2 show Cy3-images alone; C1′ and C2′ are merged images of Cy3 and GFP. Pictures in B and C are at the same magnification. (Scale bar: 10 μm.) (D) Quantitative analyses of Cy3-NGF internalization into growth cones. The y axis is the average Cy3 intensity using AFU. The signal in GFP-transfected neurons was 10.9 ± 0.8 AFU (n = 11); in Ulk1/2-RNAi-expressing neurons, it was 3.5 ± 0.5 AFU (n = 11, P < 0.001). (E) Two-color immunofluorescence staining of anti-Ulk1 (red) and anti-clathrin (green) heavy chain on a DRG growth cone. (E1) Boxed area is shown enlarged. Most green staining does not colocalize with red staining. (F) Two-color immunofluorescence staining of anti-Ulk2 (red) and anti-clathrin (green) heavy chain. A DRG growth cone is shown. (F1) Boxed area is shown enlarged. Most green staining does not colocalize with red staining, although several filopodia seem to be “yellow” (indicating partial colocalization of Ulk2 and clathrin).
Fig. 4.
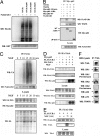
NGF stimulates ubiquitination of Ulk1 and recruitment of Ulk1 to the TrkA receptor complex. (A) K63-ubiquitinated Ulk1 interacts with p62-UBA domain in GST pull-down assay. HEK cells coexpressing FLAG-Ulk1 along with ubiquitin or its mutants were used for a p62 UBA-domain pull-down assay. The interactions were analyzed by immunoblotting with anti-HA (Ub) and GST tags. (B) Ulk1 interacts with the UBA domain of p62. Subconfluent cultures of HEK 293 cells were cotransfected as indicated. Cell lysates were immunoprecipitated with anti-myc followed by Western blotting (WB) with antibody to FLAG epitope, TRAF6, and myc to detect interaction. The lysates were Western blotted (WB) with anti-FLAG to verify the expression levels of Ulk1. (C) Time course of NGF-stimulated polyubiquitination of Ulk1. HEK cells were transfected with FLAG-tagged-Ulk1, HA-TrkA, myc-p62, and HA-Ub. Forty-eight hours after transfection, the cells were stimulated with 50 ng/ml NGF for different times (0, 5, 10, 15, 20, and 30 min) and lysed, and ubiquitination was determined by immunoprecipitation of the cell lysates with anti-FLAG antibody followed by Western blot (WB) analysis with anti-ubiquitin and FLAG. As a control, a fraction of the lysate was blotted with anti-HA and myc to check for the expression of TrkA, p62, and Ub. (D) Interaction of Ulk1 with TrkA through p62. HEK cells were transfected as indicated, stimulated with or without NGF (50 ng/ml) for 15 min followed by immunoprecipitation of HA-TrkA and Western blot (WB) with HA (TrkA), FLAG (Ulk1), and myc (p62) antibodies. The lysates were also blotted with antibody to FLAG (Ulk1), HA (TrkA), and myc (p62). Ulk1 was coimmunoprecipitated with TrkA only when p62 was present and also required NGF stimulation. (E) TrkA coimmunoprecipitated with Ulk1 in an NGF-stimulated and time-dependent manner (15 min after stimulation). (F) Ulk1 and Ulk2 can be coimmunoprecipitated with endogenous TrkA from DRG neurons, as can p62, TRAF6, and p75. Preimmune sera of anti-Ulk1 and anti-Ulk2 did not detect any bands on the same blot.
Fig. 5.
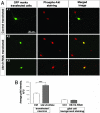
Increased phosphorylated Akt signal in Ulk1/2 knockdown neurons. (A) pAkt staining. (A1) Representative image of pAkt staining in cultures containing GFP-transfected neurons. In the same image, a transfected (GFP-positive) and a nontransfected (GFP-negative) neuron can be seen simultaneously, and the pAkt signals appear similar in both cells. (_A_2 and A3) Representative image of pAkt staining in cultures containing Ulk1/2-RNAi-transfected neurons. In both images, the transfected neuron (GFP-positive) seems to have a stronger pAkt signal than the neighboring untransfected cells. (B) Quantitative analyses of the pAkt staining intensity (AFU) averaged over the area of the cell body. The average signal in GFP-transfected neurons was 113.3 ± 8.8 AFU (n = 22); in Ulk1/2-RNAi-expressing neurons it was 211.6 ± 8.9 AFU (n = 20, P < 0.001). The background staining in glia cells was 40.3 ± 2.7 AFU for GFP-transfected cultures (_n_ = 10) and 37.7 ± 2.2 AFU (_n_ = 12, _P_ > 0.4) for Ulk1/2-RNAi-transfected cultures.
Similar articles
- Ulk1 Governs Nerve Growth Factor/TrkA Signaling by Mediating Rab5 GTPase Activation in Porcine Hemagglutinating Encephalomyelitis Virus-Induced Neurodegenerative Disorders.
Li Z, Zhao K, Lv X, Lan Y, Hu S, Shi J, Guan J, Yang Y, Lu H, He H, Gao F, He W. Li Z, et al. J Virol. 2018 Jul 31;92(16):e00325-18. doi: 10.1128/JVI.00325-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875237 Free PMC article. - The autophagy-inducing kinases, ULK1 and ULK2, regulate axon guidance in the developing mouse forebrain via a noncanonical pathway.
Wang B, Iyengar R, Li-Harms X, Joo JH, Wright C, Lavado A, Horner L, Yang M, Guan JL, Frase S, Green DR, Cao X, Kundu M. Wang B, et al. Autophagy. 2018;14(5):796-811. doi: 10.1080/15548627.2017.1386820. Epub 2017 Dec 24. Autophagy. 2018. PMID: 29099309 Free PMC article. - Semaphorin3A regulates axon growth independently of growth cone repulsion via modulation of TrkA signaling.
Ben-Zvi A, Ben-Gigi L, Yagil Z, Lerman O, Behar O. Ben-Zvi A, et al. Cell Signal. 2008 Mar;20(3):467-79. doi: 10.1016/j.cellsig.2007.10.023. Epub 2007 Nov 5. Cell Signal. 2008. PMID: 18096366 - Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.
Winckler B, Yap CC. Winckler B, et al. Traffic. 2011 Sep;12(9):1099-108. doi: 10.1111/j.1600-0854.2011.01213.x. Epub 2011 May 23. Traffic. 2011. PMID: 21535338 Free PMC article. Review. - Mitochondrial movement and positioning in axons: the role of growth factor signaling.
Chada SR, Hollenbeck PJ. Chada SR, et al. J Exp Biol. 2003 Jun;206(Pt 12):1985-92. doi: 10.1242/jeb.00263. J Exp Biol. 2003. PMID: 12756280 Review.
Cited by
- Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease.
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J. Lipinski MM, et al. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14164-9. doi: 10.1073/pnas.1009485107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660724 Free PMC article. - Conserved role for autophagy in Rho1-mediated cortical remodeling and blood cell recruitment.
Kadandale P, Stender JD, Glass CK, Kiger AA. Kadandale P, et al. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10502-7. doi: 10.1073/pnas.0914168107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498061 Free PMC article. - Ulk1 Governs Nerve Growth Factor/TrkA Signaling by Mediating Rab5 GTPase Activation in Porcine Hemagglutinating Encephalomyelitis Virus-Induced Neurodegenerative Disorders.
Li Z, Zhao K, Lv X, Lan Y, Hu S, Shi J, Guan J, Yang Y, Lu H, He H, Gao F, He W. Li Z, et al. J Virol. 2018 Jul 31;92(16):e00325-18. doi: 10.1128/JVI.00325-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875237 Free PMC article. - The requirement of uncoordinated 51-like kinase 1 (ULK1) and ULK2 in the regulation of autophagy.
Lee EJ, Tournier C. Lee EJ, et al. Autophagy. 2011 Jul;7(7):689-95. doi: 10.4161/auto.7.7.15450. Epub 2011 Jul 1. Autophagy. 2011. PMID: 21460635 Free PMC article. - ATG13: just a companion, or an executor of the autophagic program?
Alers S, Wesselborg S, Stork B. Alers S, et al. Autophagy. 2014 Jun;10(6):944-56. doi: 10.4161/auto.28987. Autophagy. 2014. PMID: 24879146 Free PMC article. Review.
References
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Neuron. 2005;46:205–217. - PubMed
- Kamiguchi H. Mol Neurobiol. 2003;28:219–228. - PubMed
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. Nat Cell Biol. 2003;5:819–826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases