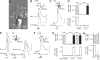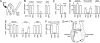The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds - PubMed (original) (raw)
Comparative Study
. 2007 Apr 10;104(15):6436-41.
doi: 10.1073/pnas.0611280104. Epub 2007 Mar 26.
Affiliations
- PMID: 17389364
- PMCID: PMC1851090
- DOI: 10.1073/pnas.0611280104
Comparative Study
The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds
Yi-Jen Huang et al. Proc Natl Acad Sci U S A. 2007.
Abstract
ATP has been shown to be a taste bud afferent transmitter, but the cells responsible for, and the mechanism of, its release have not been identified. Using CHO cells expressing high-affinity neurotransmitter receptors as biosensors, we show that gustatory stimuli cause receptor cells to secrete ATP through pannexin 1 hemichannels in mouse taste buds. ATP further stimulates other taste cells to release a second transmitter, serotonin. These results provide a mechanism to link intracellular Ca(2+) release during taste transduction to secretion of afferent transmitter, ATP, from receptor cells. They also indicate a route for cell-cell communication and signal processing within the taste bud.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Use of cellular biosensors to examine neurotransmitter secretion from receptor (Type II) and presynaptic (Type III) cells in the mouse taste bud. (A) Example of a pipette-held biosensor (ATP-Bio) apposed to an isolated taste receptor cell (Rec). Both cells were loaded with Fura-2. (B, C, E, and F insets) The recording arrangement with identified taste cell apposed to a biosensor cell. Paired traces show concurrent Ca2+ responses in a taste cell (Upper) and its apposed biosensor (Lower). Calibration bars for all traces: 20 sec and 0.5 F340/F380. (B) Taste receptor cells secrete ATP. Example of concurrent recordings of Ca2+ responses in a receptor cell (Rec) and an apposed ATP biosensor cell. Bath-applied tastants (bar at bottom of traces: a mix of 10 μM cycloheximide, 1 mM denatonium, 2 mM saccharin, and 100 μM SC45647) evoked responses in the receptor cell (upper trace) and after a brief delay, in the ATP biosensor (lower trace). (C) Presynaptic cells do not secrete ATP. Example of concurrent recordings from a presynaptic cell (Pre) and an ATP biosensor cell. KCl depolarization (50 mM) evoked a robust Ca2+ response in the presynaptic cell but did not stimulate ATP secretion. (D) Summary of recordings exemplified in B and C. Only receptor cells (Upper, gray bar) secrete ATP (Lower, white bar) and only in response to tastants, not KCl. In contrast, presynaptic cells (Upper, black bar) respond to KCl depolarization, not to taste stimulation and do not secrete ATP (Lower, white bar). Bars show means ± SEM. of Ca2+ responses, normalized to the maximum response for each stimulus (Upper), or for maximum ATP biosensor response (Lower). (E) Presynaptic cells release serotonin (5-HT) when stimulated. Example of concurrent Ca2+ responses in a presynaptic cell (upper trace) and an apposed 5-HT biosensor (lower trace) during sequential stimulation with KCl (50 mM), taste mix (as in B), and ATP (1 μM). KCl depolarization and ATP both triggered Ca2+ responses and 5-HT release. (F) Receptor cells do not release 5-HT. Example of a Ca2+ response in a receptor cell during taste stimulation (upper trace). There is no response in the apposed 5-HT biosensor cell (lower trace). (G) Summary of recordings exemplified in E and F. Only presynaptic cells (Upper, black bars) secrete 5-HT (Lower, white bars) and do so in response to KCl depolarization or ATP (1 μM) stimulation. Receptor cells (Upper, gray bars) also produce a Ca2+ response to ATP stimulation but do not secrete 5-HT (Lower). (H) Receptor cells (Upper, gray bars), stimulated with a taste mix (as in B) release ATP (Lower, white bars). In the presence of 5 μM carbenoxolone (cbx), a blocker of Px hemichannels, tastant-evoked ATP release (right) was severely diminished. ∗∗∗, P < 0.001.
Fig. 2.
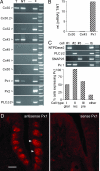
Taste cells express hemichannel-forming Px 1 subunits. (A) RT-PCR for hemichannel-forming subunits expressed in taste bud-enriched (T) or nontaste (NT) lingual epithelium, a negative control lacking template (−), and a positive control tissue (+; lens for Cx46 and Cx50, vallate papilla for PLCβ2, or brain for all others). Only Cx30, Cx43, and Px1 were prominent in the taste samples. (B) Real-time RT-PCR shows that, of the abundantly expressed hemichannel-forming subunits, only Px1 is preferentially expressed in taste buds. Copy number of mRNA for each gene was normalized to β-actin mRNA copy for each of three independent samples. (C) Expression profiling of isolated taste cells shows that Px1 is expressed in all receptor cells. Linear-amplified RNA from 51 individual cells was tested by RT-PCR for Px1 and for markers diagnostic of the three taste cell types. Gels show RT-PCRs for one representative cell of each type: a glial-like (Type I), a receptor (rec) (Type II), and a presynaptic (pre) (Type III) cell. The bar graph summarizes data on the prevalence of Px1 in each taste cell type and in cells lacking all three markers (“other”) (n = 12, 10, 10, and 19 respectively). See
SI Table 1
for complete data on all cells. (D) In situ hybridization using antisense probe (Left) on cryosections of vallate taste papilla shows a strong signal for Px1 in taste buds (arrowheads) but not when the control sense probe (Right) was used. (Scale bar, 100 μm.)
Fig. 3.
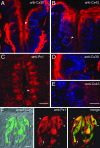
Immunostaining for Cx and Px in taste buds. (A) Cx30 immunofluorescence (red) is prominent in keratinocytes of nontaste epithelium and in nerve fibers or glia in the lamina propria. It is undetectable in taste buds (e.g., arrowhead). Nuclei stained with DAPI reveal immunonegative cells. (B) Similarly, Cx43 immunostaining is prominently found in keratinocytes and minimally, if at all, within taste buds (e.g., arrowhead). (C) In contrast, Px1-immunofluorescence is seen in most vallate taste buds (arrowhead) and, at lower intensity, in other cells in the surrounding tissue. (D) A taste bud (from A) at higher magnification, shows no immunoreactivity for Cx30, although surface keratinocytes and a nerve plexus around the taste bud are stained. (E) Higher magnification (from B) demonstrates that taste buds lack Cx43 signal, although surrounding epithelial cells are immunopositive. (F) Higher magnification of Px1 immunostaining in one taste bud. Double immunofluorescence shows that receptor cells, identified with anti-PLCβ2 (Left, green; merged with Nomarski optics) also are immunostained with anti-Px1 (Center, red). Two representative double-immunostained cells are indicated (yellow, arrowheads) in the merged image (Right). Px1 is also detected in some taste bud cells that lack PLCβ2 (arrow), consistent with cDNA profiling results in Fig. 2_C_. [Scale bars, 100 μm (A–C); 50 μm (D and E); 10 μm (F).]
Fig. 4.
Carboxyfluorescein uptake demonstrates functional Px1-like hemichannels in taste cells. (A) Validation of dye-uptake assay for Px1 hemichannels. CHO cells, control (open bars) or transfected with mouse Px1 (hatched bars), were incubated with carboxyfluorescein (5 mM) to estimate uptake at rest. Cells were stimulated with 5 μM ATP to increase [Ca2+]i and open Px1 hemichannels while dye was still present. Bars represent mean fluorescence ± SEM., normalized to maximum, ATP-stimulated fluorescence for each cell (n = 113 from three independent experiments; P ≤ 0.05). (B) Isolated mouse vallate taste cells, incubated in dye and stimulated with 5 μM ATP as in A show a broad range of ΔF/F (increase in relative fluorescence over background level) upon stimulation with ATP. (C) Mean ± SEM. (n = 52 cells) of the relative increase of fluorescence. (D–F) Confocal micrographs of isolated taste cells in the dye assay. (D) Bright-field (Nomarski optics) image of taste cells. (E) Fluorescence intensity (represented by pseudocolor) after ATP-stimulated dye uptake in the presence of 5 μM carbenoxolone (cbx) to block Px1 hemichannels. (F) Fluorescence levels in the same field of cells after cbx was washed out and cells were restimulated with ATP in presence of dye, as in E. Basal level of fluorescence (i.e., unstimulated dye uptake) was subtracted from images E and F. Examples of cells that took up dye are indicated with arrowheads. (Scale bar, 5 μm.) (G) Summary of effects of cbx on dye uptake in isolated mouse taste cells (mean ± SEM.; n = 99 cells; ∗, P ≤ 0.05).
Fig. 5.
Taste-evoked release of 5-HT from taste buds is abolished by treatments that interrupt ATP transmission within the taste bud. (A) Diagram showing stimulus/recording arrangement for the experiments in B–F. A 5-HT biosensor cell is apposed to an isolated taste bud and evoked release of 5-HT is measured under varying treatments. The biosensor cell itself was unaffected by these treatments (
SI Fig. 6
). (B) Suramin, a broad-spectrum P2 receptor antagonist, blocked 5-HT release evoked by taste stimulation (taste mixture as in Fig. 1) but not by KCl depolarization. (C) bathing taste buds in exogenous ATPase (apyrase, 10 units/ml) strongly reduced taste-evoked 5-HT release, consistent with ATP mediating cell–cell signaling. (D–F) treatments that block gap junction hemichannels eliminate taste-evoked 5-HT release. (D) Intracellular acidification produced by 20 mM Na acetate eliminated taste-evoked 5-HT release. [The 5-HT release evoked by direct stimulation of presynaptic cells by KCl depolarization or by ATP (1 μM) was also reduced, possibly because of actions of decreased cytosolic pH on voltage-gated Ca channels and second messenger cascades, respectively.] (E) Similarly, buffer equilibrated with 5% CO2 eliminated taste-evoked 5-HT release and decreased 5-HT release evoked by direct stimulation of presynaptic cells. (F) Carbenoxolone (5 μM), a potent and selective blocker of Px1 gap junction channels at this concentration, eliminated taste-evoked 5-HT release. (G) proposed model for cell–cell communication in mouse vallate taste buds. Taste stimulation of receptor cells opens Px1 hemichannels, leading to ATP secretion. Secreted ATP acts on nearby serotonergic presynaptic (Type III) cells to stimulate 5-HT release. (Because afferent fibers are absent from our experiments, we have omitted the role of ATP in stimulating sensory afferents.)
Similar articles
- Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels.
Dando R, Roper SD. Dando R, et al. J Physiol. 2009 Dec 15;587(Pt 24):5899-906. doi: 10.1113/jphysiol.2009.180083. J Physiol. 2009. PMID: 19884319 Free PMC article. - Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds.
Huang AY, Wu SY. Huang AY, et al. J Neurosci. 2015 Sep 16;35(37):12714-24. doi: 10.1523/JNEUROSCI.0100-15.2015. J Neurosci. 2015. PMID: 26377461 Free PMC article. - Afferent neurotransmission mediated by hemichannels in mammalian taste cells.
Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Romanov RA, et al. EMBO J. 2007 Feb 7;26(3):657-67. doi: 10.1038/sj.emboj.7601526. Epub 2007 Jan 18. EMBO J. 2007. PMID: 17235286 Free PMC article. - Cell communication in taste buds.
Roper SD. Roper SD. Cell Mol Life Sci. 2006 Jul;63(13):1494-500. doi: 10.1007/s00018-006-6112-9. Cell Mol Life Sci. 2006. PMID: 16732426 Free PMC article. Review. - Signal transduction and information processing in mammalian taste buds.
Roper SD. Roper SD. Pflugers Arch. 2007 Aug;454(5):759-76. doi: 10.1007/s00424-007-0247-x. Epub 2007 Apr 28. Pflugers Arch. 2007. PMID: 17468883 Free PMC article. Review.
Cited by
- Mechanisms of constitutive and ATP-evoked ATP release in neonatal mouse olfactory epithelium.
Hayoz S, Jia C, Hegg C. Hayoz S, et al. BMC Neurosci. 2012 May 28;13:53. doi: 10.1186/1471-2202-13-53. BMC Neurosci. 2012. PMID: 22640172 Free PMC article. - Acetic acid modulates spike rate and spike latency to salt in peripheral gustatory neurons of rats.
Breza JM, Contreras RJ. Breza JM, et al. J Neurophysiol. 2012 Nov;108(9):2405-18. doi: 10.1152/jn.00114.2012. Epub 2012 Aug 15. J Neurophysiol. 2012. PMID: 22896718 Free PMC article. - Cocaine decreases saccharin preference without altering sweet taste sensitivity.
Roebber JK, Izenwasser S, Chaudhari N. Roebber JK, et al. Pharmacol Biochem Behav. 2015 Jun;133:18-24. doi: 10.1016/j.pbb.2015.03.010. Epub 2015 Mar 24. Pharmacol Biochem Behav. 2015. PMID: 25812471 Free PMC article. - Endogenous pannexin1 channels form functional intercellular cell-cell channels with characteristic voltage-dependent properties.
Palacios-Prado N, Soto PA, López X, Choi EJ, Marquez-Miranda V, Rojas M, Duarte Y, Lee J, González-Nilo FD, Sáez JC. Palacios-Prado N, et al. Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2202104119. doi: 10.1073/pnas.2202104119. Epub 2022 Apr 29. Proc Natl Acad Sci U S A. 2022. PMID: 35486697 Free PMC article. - Extracellular adenosine regulates naive T cell development and peripheral maintenance.
Cekic C, Sag D, Day YJ, Linden J. Cekic C, et al. J Exp Med. 2013 Nov 18;210(12):2693-706. doi: 10.1084/jem.20130249. Epub 2013 Oct 21. J Exp Med. 2013. PMID: 24145516 Free PMC article.
References
- Margolskee RF. J Biol Chem. 2002;277:1–4. - PubMed
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. Science. 2005;310:1495–1499. - PubMed
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. J Comp Neurol. 2001;440:97–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC006308/DC/NIDCD NIH HHS/United States
- R56 DC006308/DC/NIDCD NIH HHS/United States
- R55 DC007630/DC/NIDCD NIH HHS/United States
- R01 DC007630/DC/NIDCD NIH HHS/United States
- DC006308/DC/NIDCD NIH HHS/United States
- DC007630/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous