Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions - PubMed (original) (raw)
Comparative Study
. 2007 Apr 3;104(14):6013-8.
doi: 10.1073/pnas.0610394104. Epub 2007 Mar 26.
Affiliations
- PMID: 17389368
- PMCID: PMC1851608
- DOI: 10.1073/pnas.0610394104
Comparative Study
Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions
Shotaro Suzuki et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Recent studies describing the seemingly contradictory actions of estrogens in ischemic stroke injury have led us to reevaluate the circumstances under which estrogen therapy (ET) provides benefits against cerebral stroke and decipher its mechanisms of action. One prominent feature that follows stroke injury is massive central and peripheral inflammatory responses. Evidence now suggests that postischemic inflammatory responses strongly contribute to the extent of brain injury, and 17beta-estradiol (E(2)) may protect the ischemic brain by exerting antiinflammatory actions. In an attempt to explain recently reported dichotomous effects of E(2) in stroke injury, we tested the hypothesis that an extended period of hypoestrogenicity both prevents E(2) from protecting the brain against ischemia and simultaneously suppresses its antiinflammatory actions. We report that E(2) exerts profound neuroprotective action when administered immediately upon ovariectomy, but not when administered after 10 weeks of hypoestrogenicity. Consistently, E(2) treatment given immediately at the time of ovariectomy attenuated central and peripheral production of proinflammatory cytokines after ischemic stroke. In contrast, E(2) did not suppress production of proinflammatory molecules when it was administered after 10 weeks postovariectomy. These results demonstrate that a prolonged period of hypoestrogenicity disrupts both neuroprotective and antiinflammatory actions of E(2). Our findings may help to explain the results of the Women's Health initiative that reported no beneficial effect of ET against stroke because the majority of the subjects initiated ET after an extended period of hypoestrogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
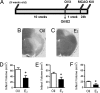
Fig. 1.
Low physiological levels of E2 protect the brain against ischemic injury. (A) C57BL/6J mice (19-week-old) were ovariectomized and immediately implanted with capsules containing either oil (n = 8) or E2 (n = 11) for 1 week. Subsequently, animals underwent experimental ischemia by middle cerebral artery occlusion (MCAO) at 20 weeks of age and were killed 24 h after the onset of injury. (B and C) Infarct volumes were measured by using TTC staining in oil- (B) or E2- (C) treated mice. (D–F) Immediate E2 treatment significantly reduced the total infarct volume (D; ∗, P < 0.02) and the extent of injury in both the cortex (E; ∗, P < 0.05) and striatum (F; ∗, P < 0.03) of 20-week-old mice (unpaired two-tailed t test, mean ± SEM, n = 8–11).
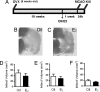
Fig. 2.
Prolonged hypoestrogenicity disrupts the ability of E2 to protect the ischemic brain. (A) Nine-week-old C57BL/6J mice were ovariectomized and 10 weeks later implanted with oil (n = 12) or E2 (n = 10) capsules for 1 week before they underwent experimental ischemia. This paradigm of E2 treatment leads to a prolonged period of hypoestrogenicity before stable serum levels of E2 are restored through a delayed implantation of a Silastic capsule. (B and C) In contrast to animals that received capsules immediately at the time of ovariectomy (Fig. 1), mice in this group exhibited extensive ischemic brain injury regardless of E2 treatment revealed by TTC staining. (D–F) A delayed E2 treatment did not reduce the total infarct volume (D; P = 0.697). Specifically, after a prolonged period of hypoestrogenicity, E2 failed to reduce brain infarction in the cortex (E; P = 0.447) as well as in the striatum (F; P = 0.581), compared with oil-treated counterparts (unpaired two-tailed t test, mean ± SEM, n = 10–12).
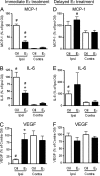
Fig. 3.
Immediate E2 treatment attenuates proinflammatory responses in the brain. In the first paradigm, mice were ovariectomized at 19 weeks of age and immediately implanted with capsules containing either oil (n = 5) or E2 (n = 8) for 1 week. (A and B) A mouse cytokine multiplex proteomic array revealed that ischemic injury increased the expression of monocyte chemoattractant protein-1 (MCP-1; #, P < 0.0001 for oil-treated mice; #, P = 0.0153 for E2-treated mice) and IL-6 (IL-6; #, P < 0.0001 for oil-treated mice; #, P = 0.0043 for E2-treated mice) on the injured side of the brain compared with the contralateral side. An immediate E2 treatment attenuated ischemia-induced up-regulation of MCP-1 (∗, P = 0.0093) and IL-6 (∗, P = 0.0271) on the ipsilateral side of the ischemic brain. (C) E2 prevented injury-induced down-regulation (#, P = 0.0004) of the neuroprotective VEGF (∗, P = 0.0024). In the second paradigm, mice at 9 weeks of age were ovariectomized for 10 weeks and subsequently implanted with an oil (n = 5) or E2 (n = 6) capsule for 1 week. (D) When administered 10 weeks after ovariectomy, E2 did not suppress (P = 0.285) the injury-induced production of MCP-1 (#, P = 0.0052). (E) Similarly, E2 did not attenuate (P = 0.541) the ischemia-induced up-regulation of IL-6 production (#, P = 0.0261). (F) A delayed E2 treatment caused no changes in the expression of VEGF (P = 0.326). In both paradigms, data represent the mean ± SEM of five to eight animals per experimental group.
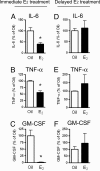
Fig. 4.
E2 exerted antiinflammatory actions only when administered immediately after ovariectomy. (A–C) Immediate E2 treatment (n = 9) suppressed plasma levels of IL-6 (A; ∗, P = 0.004), TNFα (B; ∗, P < 0.05), and GM-CSF (C; ∗, P = 0.0002), compared with oil-treated controls (n = 8). (D–F) When E2 was administered 10 weeks postovariectomy (n = 11), it did not suppress the production of IL-6 (D; P = 0.737), TNFα (E; P = 0.351), and GM-CSF (F; P = 0.663), compared with oil-treated counterparts (n = 14). In both series, data represent the mean ± SEM of 8–14 animals per experimental group.
Similar articles
- The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice.
Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ. Cai M, et al. Neurosci Lett. 2014 Jan 13;558:115-9. doi: 10.1016/j.neulet.2013.11.007. Epub 2013 Nov 15. Neurosci Lett. 2014. PMID: 24246902 - Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta.
Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Suzuki S, et al. J Comp Neurol. 2007 Feb 20;500(6):1064-75. doi: 10.1002/cne.21240. J Comp Neurol. 2007. PMID: 17183542 - Different methods for administering 17beta-estradiol to ovariectomized rats result in opposite effects on ischemic brain damage.
Strom JO, Theodorsson E, Holm L, Theodorsson A. Strom JO, et al. BMC Neurosci. 2010 Mar 17;11:39. doi: 10.1186/1471-2202-11-39. BMC Neurosci. 2010. PMID: 20236508 Free PMC article. - Neuroprotective effects of estrogens following ischemic stroke.
Suzuki S, Brown CM, Wise PM. Suzuki S, et al. Front Neuroendocrinol. 2009 Jul;30(2):201-11. doi: 10.1016/j.yfrne.2009.04.007. Epub 2009 May 3. Front Neuroendocrinol. 2009. PMID: 19401209 Free PMC article. Review. - Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies.
Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, Rosewell KL. Wise PM, et al. Brain Res Brain Res Rev. 2001 Nov;37(1-3):313-9. doi: 10.1016/s0165-0173(01)00136-9. Brain Res Brain Res Rev. 2001. PMID: 11744096 Review.
Cited by
- Treatment with brain specific estrogen prodrug ameliorates cognitive effects of surgical menopause in mice.
Salinero AE, Abi-Ghanem C, Venkataganesh H, Sura A, Smith RM, Thrasher CA, Kelly RD, Hatcher KM, NyBlom V, Shamlian V, Kyaw NR, Belanger KM, Gannon OJ, Stephens SBZ, Zuloaga DG, Zuloaga KL. Salinero AE, et al. Horm Behav. 2024 Aug;164:105594. doi: 10.1016/j.yhbeh.2024.105594. Epub 2024 Jun 24. Horm Behav. 2024. PMID: 38917776 - Estrogen's sex-specific effects on ischemic cell death and estrogen receptor mRNA expression in rat cortical organotypic explants.
Trout AL, McLouth CJ, Westberry JM, Sengoku T, Wilson ME. Trout AL, et al. Aging Brain. 2024 Apr 16;5:100117. doi: 10.1016/j.nbas.2024.100117. eCollection 2024. Aging Brain. 2024. PMID: 38650743 Free PMC article. - The role of the brain renin-angiotensin system in Parkinson´s disease.
Labandeira-Garcia JL, Labandeira CM, Guerra MJ, Rodriguez-Perez AI. Labandeira-Garcia JL, et al. Transl Neurodegener. 2024 Apr 15;13(1):22. doi: 10.1186/s40035-024-00410-3. Transl Neurodegener. 2024. PMID: 38622720 Free PMC article. Review. - Talking about Sexuality in Stroke Individuals: The New Era of Sexual Rehabilitation.
Contrada M, Cerasa A, Pucci C, Ciancarelli I, Pioggia G, Tonin P, Calabrò RS. Contrada M, et al. J Clin Med. 2023 Jun 12;12(12):3988. doi: 10.3390/jcm12123988. J Clin Med. 2023. PMID: 37373681 Free PMC article. - Are the digit ratio (2D:4D) and hand grip strength related to Parkinson disease in elderly males?
Arazi H, Birak Olia RB, Eghbali E. Arazi H, et al. BMC Sports Sci Med Rehabil. 2023 Mar 20;15(1):34. doi: 10.1186/s13102-023-00642-2. BMC Sports Sci Med Rehabil. 2023. PMID: 36941653 Free PMC article.
References
- Bushnell CD. Semin Neurol. 2006;26:123–130. - PubMed
- Mitka M. JAMA. 2006;295:1755–1756. - PubMed
- Paganini-Hill A. Maturitas. 2001;38:243–261. - PubMed
- Turgeon JL, McDonnell DP, Martin KA, Wise PM. Science. 2004;304:1269–1273. - PubMed
- Behl C. Nat Rev Neurosci. 2002;3:433–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG02224/AG/NIA NIH HHS/United States
- R37 AG002224/AG/NIA NIH HHS/United States
- AG17164/AG/NIA NIH HHS/United States
- P01 AG017164/AG/NIA NIH HHS/United States
- R01 AG002224/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources