Micromechanical control of cell-cell interactions - PubMed (original) (raw)
Micromechanical control of cell-cell interactions
Elliot E Hui et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The development and function of living tissues depends largely on interactions between cells that can vary in both time and space; however, temporal control of cell-cell interaction is experimentally challenging. By using a micromachined silicon substrate with moving parts, we demonstrate the dynamic regulation of cell-cell interactions via direct manipulation of adherent cells with micrometer-scale precision. We thereby achieve mechanical control of both tissue composition and spatial organization. As a case study, we demonstrate the utility of this tool in deconstructing the dynamics of intercellular communication between hepatocytes and supportive stromal cells in coculture. Our findings indicate that the maintenance of the hepatocellular phenotype by stroma requires direct contact for a limited time ( approximately hours) followed by a sustained soluble signal that has an effective range of <400 microm. This platform enables investigation of dynamic cell-cell interaction in a multitude of applications, spanning embryogenesis, homeostasis, and pathogenic processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
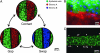
Micromechanical substrates enable micrometer-resolution cell positioning. (A) Microfabricated silicon parts can be fully separated (Left), locked together with comb fingers in contact (Center), or slightly separated (Right). Cells are cultured on the top surfaces; manual scraping can be used to restrict cells to the comb fingers only (Inset). The slope of the tapered comb fingers results in a 20:1 mechanical transmission ratio; that is, sliding the parts 1.6 mm changes the gap between the fingers by only 80 μm. Together with the integrated snap-lock mechanism, it is thereby possible to control separation with repeatable micrometer-scale precision by using unassisted manual actuation. (B and C) Bright-field images of hepatocytes (darker cells) and 3T3 fibroblasts cultured on the comb fingers. The silicon is first functionalized by spin-coating with polystyrene followed by plasma treatment, resulting in a surface comparable to tissue culture plastic. Devices can be reused multiple times (>20). (D) Devices in a standard 12-well plate. Cell culture and functional assays were performed with standard methods. Actuation is also performed directly on the plate with sterile tweezers.
Fig. 2.
Reconfigurable cell culture. Cultures can be reversibly switched to initiate or to eliminate contact between two cell populations; individual populations can also be removed and replaced. (A) Fluorescent images illustrating possible device manipulations. Each cell type was prelabeled with an individual dye color. (B) Fluorescent image showing intimate contact between hepatocytes (green) and stroma (red, 3T3 fibroblasts) at the interface between neighboring comb fingers. The image was taken 18 h after initiation of contact. Cell nuclei are counterstained in blue. (C) Cross-migration of cells is minimal for moderate durations of contact. Representative fluorescent image showing small numbers of stromal cells (red, arrows indicate selected cells) remaining behind on a hepatocyte finger (green) after combs were separated after 18 h of contact. In this work, contact was limited to 18 h to minimize cross-migration, but longer durations are possible with other cell types (data not shown).
Fig. 3.
Dynamic regulation of hepatocyte–stromal interactions reveals temporal dependencies in intercellular communication. (A) Contact between hepatocyte and fibroblast combs was required to maintain albumin secretion over a 2-wk period (red). In the gap mode (blue), function dropped almost as rapidly as with hepatocytes alone (green). (B) An 18-h period of transient initial contact followed by long-term culture in the gap mode (which allows diffusion of paracrine signals) resulted in sustained liver-specific function (blue) similar to that obtained with sustained contact (red). However, 18 h of initial contact followed by removal of adjacent stroma resulted in deterioration of function (green). (C) After 18 h of initial contact, stroma were removed and replaced by naïve stroma (in gap mode). Liver-specific function was maintained at similar levels (blue) to that obtained with no cell swapping (red). In a parallel experiment in which naïve hepatocytes were substituted, liver-specific function was not maintained (green).
Fig. 4.
Spatial reconfiguration reveals short-range soluble signaling. (A) After 18 h of initial contact, hepatocytes and stroma were separated into individual wells. Stromal conditioned medium was transferred every 2 days to the hepatocytes, but liver-specific function declined (blue). In contrast, transient contact followed by microscale separation (using the gap mode) resulted in sustained function (red). (B) Loss in liver-specific function progresses to loss in hepatocyte viability. Hepatocyte viability was probed by using a membrane integrity dye (calcein AM, green) with a nuclear counterstain for both cell types (blue). After initial contact, cultures were maintained for 2 weeks in the gap mode, resulting in a sharp gradient in hepatocyte viability dependent on proximity to stroma (n > 3; representative image shown). Selected comb fingers are outlined in white for clarity. (C) Quantified calcein fluorescence along the length of a comb finger (n = 9). L, the characteristic decay length of viability, is measured to be 325 μm by using an exponential fit over x > 0.
Fig. 5.
Proposed model for intercellular communication. Maintenance of liver-specific function in hepatocytes requires an initial short-term (τ ≈ 18 h) contact-mediated signal from stromal cells (Upper), followed by sustained short-range (L ≈ 325 μm) soluble signaling from the stroma (Lower).
Similar articles
- Microfabrication of a tunable collagen/alginate-chitosan hydrogel membrane for controlling cell-cell interactions.
Song Y, Zhang D, Lv Y, Guo X, Lou R, Wang S, Wang X, Yu W, Ma X. Song Y, et al. Carbohydr Polym. 2016 Nov 20;153:652-662. doi: 10.1016/j.carbpol.2016.07.058. Epub 2016 Jul 18. Carbohydr Polym. 2016. PMID: 27561537 - Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance.
Lu HF, Chua KN, Zhang PC, Lim WS, Ramakrishna S, Leong KW, Mao HQ. Lu HF, et al. Acta Biomater. 2005 Jul;1(4):399-410. doi: 10.1016/j.actbio.2005.04.003. Epub 2005 Jun 13. Acta Biomater. 2005. PMID: 16701821 - Silicon microchips for manipulating cell-cell interaction.
Hui EE, Bhatia SN. Hui EE, et al. J Vis Exp. 2007;(7):268. doi: 10.3791/268. Epub 2007 Aug 30. J Vis Exp. 2007. PMID: 18989439 Free PMC article. - Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling.
Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. Khetani SR, et al. Hepatology. 2004 Sep;40(3):545-54. doi: 10.1002/hep.20351. Hepatology. 2004. PMID: 15349892 - Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions.
Bhatia SN, Balis UJ, Yarmush ML, Toner M. Bhatia SN, et al. Biotechnol Prog. 1998 May-Jun;14(3):378-87. doi: 10.1021/bp980036j. Biotechnol Prog. 1998. PMID: 9622518
Cited by
- Engineering cell-cell signaling.
Blagovic K, Gong ES, Milano DF, Natividad RJ, Asthagiri AR. Blagovic K, et al. Curr Opin Biotechnol. 2013 Oct;24(5):940-947. doi: 10.1016/j.copbio.2013.05.007. Epub 2013 Jul 12. Curr Opin Biotechnol. 2013. PMID: 23856592 Free PMC article. Review. - Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences.
Zhang P, Su J, Mende U. Zhang P, et al. Am J Physiol Heart Circ Physiol. 2012 Dec 15;303(12):H1385-96. doi: 10.1152/ajpheart.01167.2011. Epub 2012 Oct 12. Am J Physiol Heart Circ Physiol. 2012. PMID: 23064834 Free PMC article. Review. - Cellular and multicellular form and function.
Liu WF, Chen CS. Liu WF, et al. Adv Drug Deliv Rev. 2007 Nov 10;59(13):1319-28. doi: 10.1016/j.addr.2007.08.011. Epub 2007 Aug 16. Adv Drug Deliv Rev. 2007. PMID: 17884241 Free PMC article. Review. - Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions.
Kaji H, Camci-Unal G, Langer R, Khademhosseini A. Kaji H, et al. Biochim Biophys Acta. 2011 Mar;1810(3):239-50. doi: 10.1016/j.bbagen.2010.07.002. Epub 2010 Jul 23. Biochim Biophys Acta. 2011. PMID: 20655984 Free PMC article. Review. - Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer.
Kogure T, Yan IK, Lin WL, Patel T. Kogure T, et al. Genes Cancer. 2013 Jul;4(7-8):261-72. doi: 10.1177/1947601913499020. Genes Cancer. 2013. PMID: 24167654 Free PMC article.
References
- El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442:403–411. - PubMed
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883–1900. - PubMed
- Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Biomaterials. 1995;16:297–303. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials