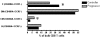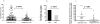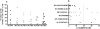Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection - PubMed (original) (raw)
doi: 10.1371/journal.pone.0000321.
Rika Draenert, Almas Rathod, Cori L Verrill, Benjamin T Davis, Rajesh T Gandhi, Gregory K Robbins, Nesli O Basgoz, David R Stone, Daniel E Cohen, Mary N Johnston, Theresa Flynn, Alysse G Wurcel, Eric S Rosenberg, Marcus Altfeld, Bruce D Walker
Affiliations
- PMID: 17389912
- PMCID: PMC1824710
- DOI: 10.1371/journal.pone.0000321
Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection
Marylyn M Addo et al. PLoS One. 2007.
Abstract
Background: CD8+ T cells impact control of viral infections by direct elimination of infected cells and secretion of a number of soluble factors. In HIV-1 infection, persistent HIV-1 specific IFN-gamma+ CD8+ T cell responses are detected in the setting of disease progression, consistent with functional impairment in vivo. Recent data suggest that impaired maturation, as defined by the lineage markers CD45RA and CCR7, may contribute to a lack of immune control by these responses.
Methodology/principal findings: We investigated the maturation phenotype of epitope-specific CD8+ T cell responses directed against HIV-1 in 42 chronically infected, untreated individuals, 22 of whom were "Controllers" (median 1140 RNA copies/ml plasma, range<50 to 2520), and 20 "progressors" of whom had advanced disease and high viral loads (median 135,500 RNA copies/ml plasma, range 12100 to >750000). Evaluation of a mean of 5 epitopes per person revealed that terminally differentiated CD8+ T cells directed against HIV-1 are more often seen in HIV-1 Controllers (16/22; 73%) compared to HIV-1 progressors (7/20; 35%)(p = 0.015), but the maturation state of epitope-specific responses within a given individual was quite variable. Maturation phenotype was independent of the HLA restriction or the specificity of a given CD8+ T cell response and individual epitopes associated with slow disease progression were not more likely to be terminally differentiated.
Conclusions/significance: These data indicate that although full maturation of epitope-specific CD8+ T cell responses is associated with viral control, the maturation status of HIV-1 specific CD8+ T cell responses within a given individual are quite heterogeneous, suggesting epitope-specific influences on CD8+ T cell function.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. T cell differentiation profile in bulk CD8+ T cells.
Based on the lineage markers, CD45RA and CCR7 bulk CD8+ T cells of HIV-1 Controllers (black bars) and HIV-1 progressors (hatched bars) were stratified into 4 subpopulations: effector (E), effector memory (EM), central memory (CM) and naïve (N) CD8+ T cells. Terminally differentiated (E) CD8+ T cells were of significantly higher frequency in HIV-1 Controllers (p = 0.017, Mann-Whitney), while Progressors had higher levels of CM cells (p = 0.02), indicated by asterisks (*).
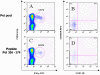
Figure 2. The effector phenotype of an individual CD8+T cell response may not be adequately reflected in a peptide pool.
Panels A and C show IFN-γ production upon stimulation with the Pol peptide pool (A) and an individual Pol peptide (C). Panels B and D are gated on the IFN-γ producing cells and show differentiation phenotype of these HIV-1 specific cells as defined by CCR7 and CD45RA isoform. The terminally differentiated response to the Pol peptide 356–374 (31.9% CCR7−/CD45RA+ of gated cells) is diluted in the response to the entire Pol peptide pool (only 8.8% gated cells are CCR7−/CD45RA+).
Figure 3. Terminally differentiated HIV-1-speficic CD8+ T cells are more frequently detectable in HIV-1 Controllers compared to HIV-1 Progressors.
(A) Percentage of CD45RA+/CCR7−/CD8+ T cells for the 126 and 106 HIV-1-specific CD8+ T cell responses tested in HIV-1 Controllers and Progressors, respectively. Frequencies of effector phenotype T cell responses were significantly higher in HIV-1 Controllers (squares) compared to progressors (triangles). An HIV-1 specific CD8+ T cell response with >20% of CD45RA+/CCR7− cells was considered to be of terminally differentiated phenotype (dotted line) . (B) More HIV-1 Controllers had at least one terminally differentiated HIV-1 specific T cell response compared to HIV-1 Progressors (p = 0.015, Fisher's exact). (C) Of the CD8+ T cell responses against HIV-1 tested per individual a higher percentage of terminally differentiated responses were detectable in HIV-1 Controllers (p = 0.007, Mann Whitney). For 3 Controllers all investigated responses were of the terminally differentiated phenotype, while five Controllers had none.
Figure 4. Maturation phenotype of HIV-1 specific CD8+ T cells by HLA type and epitope specificity.
Panel A shows the percentage of CD45RA+/CCR7− CD8+ T cells of 46 HLA-A2, -A3, -B14, -B27 and -B57-restricted epitopes tested in the study cohort. Panel B depicts the percentage of CD45RA+/CCR7− CD8+ T cells specific for six specific HIV-1 epitopes tested (restricting HLA allele and peptide sequence are shown). Data reflective of a total of 21 study subjects, who had responses to the specific HLA-matched optimal epitopes tested (14 Controllers and 5 Progressors).
Similar articles
- HIV replication leads to skewed maturation of CD8-positive T-cell responses in infected children.
Montesano C, Anselmi A, Palma P, Bernardi S, Cicconi R, Mattei M, Castelli-Gattinara G, Ciccozzi M, Colizzi V, Amicosante M. Montesano C, et al. New Microbiol. 2010 Oct;33(4):303-9. New Microbiol. 2010. PMID: 21213588 - Characterization of virus-specific CD8(+) effector T cells in the course of HIV-1 infection: longitudinal analyses in slow and rapid progressors.
Jansen CA, Piriou E, Bronke C, Vingerhoed J, Kostense S, van Baarle D, Miedema F. Jansen CA, et al. Clin Immunol. 2004 Dec;113(3):299-309. doi: 10.1016/j.clim.2004.08.002. Clin Immunol. 2004. PMID: 15507395 - Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment.
Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, Martin JN, Nixon DF, McCune JM, Deeks SG. Emu B, et al. J Virol. 2005 Nov;79(22):14169-78. doi: 10.1128/JVI.79.22.14169-14178.2005. J Virol. 2005. PMID: 16254352 Free PMC article. - Failing immune control as a result of impaired CD8+ T-cell maturation: CD27 might provide a clue.
van Baarle D, Kostense S, van Oers MH, Hamann D, Miedema F. van Baarle D, et al. Trends Immunol. 2002 Dec;23(12):586-91. doi: 10.1016/s1471-4906(02)02326-8. Trends Immunol. 2002. PMID: 12464570 Review. - Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection.
Lieberman J, Shankar P, Manjunath N, Andersson J. Lieberman J, et al. Blood. 2001 Sep 15;98(6):1667-77. doi: 10.1182/blood.v98.6.1667. Blood. 2001. PMID: 11535496 Review.
Cited by
- The role of TEMRA cell-mediated immune senescence in the development and treatment of HIV disease.
Guo L, Liu X, Su X. Guo L, et al. Front Immunol. 2023 Oct 12;14:1284293. doi: 10.3389/fimmu.2023.1284293. eCollection 2023. Front Immunol. 2023. PMID: 37901239 Free PMC article. Review. - The latency-reversing agent HODHBt synergizes with IL-15 to enhance cytotoxic function of HIV-specific T cells.
Copertino DC Jr, Holmberg CS, Weiler J, Ward AR, Howard JN, Levinger C, Pang AP, Corley MJ, Dündar F, Zumbo P, Betel D, Gandhi RT, McMahon DK, Bosch RJ, Linden N, Macatangay BJ, Cyktor JC, Eron JJ, Mellors JW, Kovacs C, Benko E, Bosque A, Jones RB; AIDS Clinical Trials Group (ACTG) A5321 Team. Copertino DC Jr, et al. JCI Insight. 2023 Sep 22;8(18):e169028. doi: 10.1172/jci.insight.169028. JCI Insight. 2023. PMID: 37581929 Free PMC article. - The role of CD38 in HIV infection.
Lu L, Wang J, Yang Q, Xie X, Huang Y. Lu L, et al. AIDS Res Ther. 2021 Apr 5;18(1):11. doi: 10.1186/s12981-021-00330-6. AIDS Res Ther. 2021. PMID: 33820568 Free PMC article. Review. - Evaluation of antiviral T cell responses and TSCM cells in volunteers enrolled in a phase I HIV-1 subtype C prophylactic vaccine trial in India.
Munusamy Ponnan S, Hayes P, Fernandez N, Thiruvengadam K, Pattabiram S, Nesakumar M, Srinivasan A, Kathirvel S, Shankar J, Goyal R, Singla N, Mukherjee J, Chatrath S, Gilmour J, Subramanyam S, Prasad Tripathy S, Swaminathan S, Hanna LE. Munusamy Ponnan S, et al. PLoS One. 2020 Feb 25;15(2):e0229461. doi: 10.1371/journal.pone.0229461. eCollection 2020. PLoS One. 2020. PMID: 32097435 Free PMC article. Clinical Trial. - Deletion of Vaccinia Virus A40R Gene Improves the Immunogenicity of the HIV-1 Vaccine Candidate MVA-B.
Pérez P, Marín MQ, Lázaro-Frías A, Sorzano CÓS, Gómez CE, Esteban M, García-Arriaza J. Pérez P, et al. Vaccines (Basel). 2020 Feb 6;8(1):70. doi: 10.3390/vaccines8010070. Vaccines (Basel). 2020. PMID: 32041218 Free PMC article.
References
- Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. - PubMed
- Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4:630–640. - PubMed
- Draenert R, Goebel FD. What's new in HIV/AIDS. Protective immunity in HIV infection: where do we stand? Infection. 2004;32:250–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI28568/AI/NIAID NIH HHS/United States
- N01-A1-15442/PHS HHS/United States
- R01 AI054178/AI/NIAID NIH HHS/United States
- Howard Hughes Medical Institute/United States
- R01 AI028568/AI/NIAID NIH HHS/United States
- R56 AI054178/AI/NIAID NIH HHS/United States
- AI054178-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials