Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance - PubMed (original) (raw)
Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance
Andrei E Medvedev et al. J Biol Chem. 2007.
Abstract
In this study, we examined whether tyrosine phosphorylation of the Toll-IL-1 resistance (TIR) domain of Toll-like receptor (TLR) 4 is required for signaling and blocked in endotoxin tolerance. Introduction of the P712H mutation, responsible for lipopolysaccharide (LPS) unresponsiveness of C3H/HeJ mice, into the TIR domain of constitutively active mouse DeltaTLR4 and mutation of the homologous P714 in human CD4-TLR4 rendered them signaling-incompetent and blocked TLR4 tyrosine phosphorylation. Mutations of tyrosine residues Y674A and Y680A within the TIR domains of CD4-TLR4 impaired its ability to elicit phosphorylation of p38 and JNK mitogen-activated protein kinases, IkappaB-alpha degradation, and activation of NF-kappaB and RANTES reporters. Likewise, full-length human TLR4 expressing Y674A or Y680A mutations showed suppressed capacities to mediate LPS-inducible cell activation. Signaling deficiencies of the Y674A and Y680A TLR4s correlated with altered MyD88-TLR4 interactions, increased associations with a short IRAK-1 isoform, and decreased amounts of activated IRAK-1 in complex with TLR4. Pretreatment of human embryonic kidney (HEK) 293/TLR4/MD-2 cells with protein tyrosine kinase or Src kinase inhibitors suppressed LPS-driven TLR4 tyrosine phosphorylation, p38 and NF-kappaB activation. TLR2 and TLR4 agonists induced TLR tyrosine phosphorylation in HEK293 cells overexpressing CD14, MD-2, and TLR4 or TLR2. Induction of endotoxin tolerance in HEK293/TLR4/MD-2 transfectants and in human monocytes markedly suppressed LPS-mediated TLR4 tyrosine phosphorylation and recruitment of Lyn kinase to TLR4, but did not affect TLR4-MD-2 interactions. Thus, our data demonstrate that TLR4 tyrosine phosphorylation is important for signaling and is impaired in endotoxin-tolerant cells, and suggest involvement of Lyn kinase in these processes.
Figures
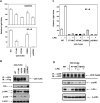
Fig. 1. The P712H mutation in the TIR domain of mouse HA-ΔTLR4 abolishes TLR4 signaling and tyrosine phosphorylation
HEK293T cells were transiently transfected with expression vectors encoding HA-tagged ΔTLR4 WT, P712H mutant, or a control vector pCDNA3. After 48 h, total RNA was isolated to examine IL-8 mRNA levels by real-time PCR (A), cell-free supernatants were collected to determine IL-8 production (B), and cell extracts prepared for Western analysis of IRAK-1, IκB-α, p38 phosphorylation, and tubulin expression (C). TLR4 species were also immunoprecipitated with α-HA Ab and subjected to immunoblotting with α-HA Ab (total TLR4 expression) and α-phosphotyrosine Ab (TLR4 phosphorylation) (D). Results of a representative experiment (n=3) are shown.
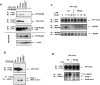
Fig. 2. The effect of the PGV714−716 mutation in the TIR domain of human CD4-TLR4 on cell activation and TLR4 tyrosine phosphorylation
CD4-TLR4 WT or PGVP714−716AAA mutant (designated P714A) was overexpressed in HEK293T cells, and transfection with pCDNA3 was used as a control. After 48 h, IL-8 gene expression was examined by real-time PCR (A), IL-8 production was determined in supernatants by ELISA (B), and Western analysis of IRAK-1 phosphorylation, IκB-α degradation, and p38 phosphorylation was performed in cellular extracts. Tubulin expression was assessed as a loading control (C). CD4-TLR4 was immunoprecipitated with α-CD4 Ab and analyzed by immunoblotting with α-Flag Ab (total TLR4 expression) and α-phosphotyrosine Ab (TLR4 phosphorylation) (D). Data from a representative experiment (n=4) are shown.
Fig. 3. Tyrosine-alanine substitutions in the TIR domain impair TLR4-induced signaling
(A) and (B): HEK293T cells were transiently transfected with expression vectors encoding either CD4-TLR4 WT, CD4-TLR4 P714H mutant, or CD4-TLR4 species expressing Y674A and Y680A mutations. As a control, pCDNA3 was transfected. (A): NF-κB (pELAM-lucifearse) and RANTES (pGL3-RANTES-lucifearse) reporters were co-expressed along with pCMV-β-galactosidase reporter, cells were recovered for 24h, and luciferase vs. β-galactosidase activities were measured. *p<0.005, **p<0.05 reflect statistically significant differences in reporter activation between CD4-TLR4 WT and the indicated mutants. (B): TLR4 species were immunoprecipitated with α-CD4 Ab and subjected to immunoblotting with α-Flag Ab (total TLR4 expression) and α-phosphotyrosine Ab (TLR4 phosphorylation). (C) and (D): Full-length WT, P714H, and Y674A TLR4 species were overexpressed in HEK293T cells along with CD14 and MD-2, in control cultures, pcDNA3 transfection was carried out. (C): NF-κB (pELAM-lucifearse) and pCMV-β-galactosidase reporters were co-transfected, and cells were stimulated with medium or 100 ng/ml LPS for 24 h. Luciferase vs. β-galactosidase activities were measured in cell extracts. (D): After recovery for 24 h, YFP-TLR4 proteins were immunoprecipitated with α-GFP Ab, and total TLR4 expression was examined by immunoblotting with α-GFP Ab. (B) and (D): Cell lysates were subjected to Western analysis of JNK and p38 phosphorylation and IκB-α degradation. β-actin immunoblots show a loading control. The middle band in the JNK immunoblot represents an unspecific band. Results of a representative experiment (n=5) are presented.
Fig. 4. The effect of the Y674A and Y680A mutations on constitutive (CD4-TLR) and LPS-inducible (full-length YFP-TLR4) interactions of TLR4 with MyD88 and IRAK-1 species
WT, Y674A and Y680A variants of CD4-TLR4 were overexpressed in HEK293T cells with (A) or without (B) YFP-MyD88. CD4-TLR4 species were immunoprecipitated with α-Flag-agarose, and TLR4 interactions with YFP-MyD88 (A) or IRAK-1 (B) were examined using α-GFP or α-IRAK-1 Abs. Total CD4-TLR4 expression was measured by immunoblot analysis of TLR4 immune complexes with α-Flag-HRP, and YFP-MyD88 expression was estimated in immunoprecipitated YFP-MyD88 using α-GFP Ab. (C) and (D): WT, Y674A YFP-TLR4 variants were co-expressed in HEK293T cells along with CD14 and MD-2. LPS stimulation was carried out as indicated (C) or for 5 min (D). YFP-TLR4s or endogenous MyD88 were immunoprecipitated, and TLR4, endogenous MyD88 and IRAK-1 expression was analyzed by immunoblotting with α-GFP, α-MyD88, and α-IRAK-1 Abs, respectively. Results of a representative experiment (n=4) are shown.
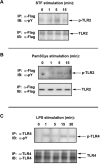
Fig. 5. TLR2 and TLR4 agonists induce tyrosine phosphorylation of respective TLRs
HEK293T cells were transiently transfected with pCDNA3-huCD14, pEFBOS-HA-huMD-2, along with either pCMV-1-Flag-huTLR2 (A,B) or pCDNA3-huTLR4 (C). Cells were recovered for 24 h, followed by stimulation with TLR2 (STF and Pam3Cys, A and B) and TLR4 (LPS, C) agonists. TLR2 and TLR4 were immunoprecipitated from cell extracts with α-Flag and α-TLR4 H80 Ab, respectively, and subjected to immunoblot analysis for total TLR2 (α-Flag Ab) or TLR4 (H80) expression, as well as TLR tyrosine phosphorylation (α-phosphotyrosine Ab PY20). Shown are data of one out of three experiments.
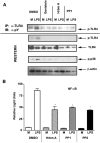
Fig. 6. The effect of protein tyrosine kinase and Src kinase inhibitors on TLR4 phosphorylation and signal transduction
HEK293/TLR4/MD-2 stable transfectants were pretreated for 45 min with genistein (100 μM), herbimycin A (1 μM), PP1 and PP2 (10 μM each), followed by stimulation with LPS for 15 min (A) or 6 h (B). In NF-κB reporter assays (B), NF-κB (pELAM-luciferase) and β-galactosidase (pCMV-β-galactosidase) reporters were co-transfected. (A): TLR4 tyrosine phosphorylation was analyzed in TLR4 immune complexes and total cell lysates vs. TLR4 total expression using α-phosphotyrosine (PY20) and α-TLR4 Abs. LPS-inducible phosphorylation of p38 was measured with α-phosphop38 Ab, and equal protein loading was controlled by running β-actin immunoblot. (B). NF-κB reporter activation was examined in cell extracts by analysis of luciferase vs. β-galactosidase activities. Shown are results of a representative (n=4) experiments.
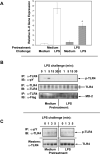
Fig. 7. Endotoxin tolerance induction inhibits TLR4 tyrosine phosphorylation in HEK293/TLR4/MD-2 stable transfectants but does not affect TLR4-MD-2 interactions
Cells were pretreated with for 20 h medium or 10 ng/ml LPS, followed by washing and restimulation with 100 ng/ml LPS as shown. (A): RNA was isolated, reverse-transcribed, and analyzed by real-time PCR for IL-8 gene expression. *p<0.05. (B) TLR4 proteins were immunoprecipitated from cell extracts with α-TLR4 Ab H80 (B) or α-phosphotyrosine Ab PY20 (C). TLR4 immune complexes (B) were analyzed with α-phosphotyrosine Ab PY20 (TLR4 phosphorylation), α-TLR4 Ab H80 (TLR4 total expression), or α-Flag Ab to estimate amounts of Flag-MD-2 associated with TLR4. Immunoprecipitated phosphotyrosine proteins (C) were subjected to immunoblot analysis using α-TLR4 Ab H80. Total cellular extracts were analyzed by immunoblotting with α-TLR4 antiserum to measure total TLR4 protein expression (C). Results of a representative experiment (n=4) are depicted.
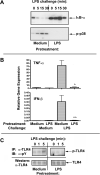
Fig. 8. Suppressed LPS-inducible tyrosine phosphorylation of TLR4 in endotoxin-tolerant human monocytes
Human monocytes were pretreated for 20 h with medium or 10 ng/ml LPS, washed and restimulated with 100 ng/ml LPS over time courses (A and C), or for 1h (IFN-β mRNA induction) and 3 h (TNF-α gene expression, B). *p<0.005; **p<0.05. Cellular extracts were examined by immunoblotting for IκB-α degradation and p38 phosphorylation (A). B: RNA was isolated and subjected to reverse transcription and real-time PCR analysis of TNF-α and IFN-β mRNA levels. (C) Cellular extracts were subjected to immunoprecipitation with α-TLR4 Ab H80 followed by immunoblotting with α-phosphotyrosine Ab PY20 (TLR4 tyrosine phosphorylation). Total TLR4 expression was analyzed by immunoblotting with α-TLR4 antiserum. Results of a representative experiment (N =7) are shown.
Fig. 9. LPS-induced recruitment of Lyn to TLR4 is suppressed in LPS-tolerant HEK/TLR4/MD-2 cells
Flag-Lyn was overexpressed in HEK293/TLR4/MD-2 stable transfectants, cells pretreated for 20 h with medium or 10 ng/ml LPS, followed by LPS stimulation as shown. Recruitment of Flag-Lyn to TLR4 was examined in TLR4 immunoprecipitates by immunoblotting with α-Flag-HRP or in Flag-Lyn immunoprecipitates immunoblotted with α-TLR4 antibody. Total expression of Flag-Lyn was measured in Lyn immune complexes with α-Flag-HRP, and total TLR4 expression was determined by immunoblotting of cellular extracts with α-TLR4 antibody. β-actin immunoblot was used to control for protein loading. The results of a representative experiment (n=3) are presented.
Similar articles
- Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling.
Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Piao W, et al. J Leukoc Biol. 2009 Oct;86(4):863-75. doi: 10.1189/jlb.0309189. Epub 2009 Aug 5. J Leukoc Biol. 2009. PMID: 19656901 Free PMC article. - Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells.
Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Medvedev AE, et al. J Immunol. 2002 Nov 1;169(9):5209-16. doi: 10.4049/jimmunol.169.9.5209. J Immunol. 2002. PMID: 12391239 - Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4.
Medvedev AE, Henneke P, Schromm A, Lien E, Ingalls R, Fenton MJ, Golenbock DT, Vogel SN. Medvedev AE, et al. J Immunol. 2001 Aug 15;167(4):2257-67. doi: 10.4049/jimmunol.167.4.2257. J Immunol. 2001. PMID: 11490013 - Molecular mechanisms of endotoxin tolerance.
Fan H, Cook JA. Fan H, et al. J Endotoxin Res. 2004;10(2):71-84. doi: 10.1179/096805104225003997. J Endotoxin Res. 2004. PMID: 15119998 Review. - Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling.
Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Płóciennikowska A, et al. Cell Mol Life Sci. 2015 Feb;72(3):557-581. doi: 10.1007/s00018-014-1762-5. Epub 2014 Oct 22. Cell Mol Life Sci. 2015. PMID: 25332099 Free PMC article. Review.
Cited by
- Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal.
De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. De S, et al. Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9680-5. doi: 10.1073/pnas.1511794112. Epub 2015 Jul 20. Proc Natl Acad Sci U S A. 2015. PMID: 26195767 Free PMC article. - Traditional biochemical assays for studying toll-like receptor 9.
Leifer CA, Rose WA 2nd, Botelho F. Leifer CA, et al. J Immunoassay Immunochem. 2013;34(1):1-15. doi: 10.1080/15321819.2012.666222. J Immunoassay Immunochem. 2013. PMID: 23323977 Free PMC article. Review. - R753Q polymorphism inhibits Toll-like receptor (TLR) 2 tyrosine phosphorylation, dimerization with TLR6, and recruitment of myeloid differentiation primary response protein 88.
Xiong Y, Song C, Snyder GA, Sundberg EJ, Medvedev AE. Xiong Y, et al. J Biol Chem. 2012 Nov 2;287(45):38327-37. doi: 10.1074/jbc.M112.375493. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992740 Free PMC article. - Gender Difference in Damage-Mediated Signaling Contributes to Pulmonary Arterial Hypertension.
Rafikov R, Nair V, Sinari S, Babu H, Sullivan JC, Yuan JX, Desai AA, Rafikova O. Rafikov R, et al. Antioxid Redox Signal. 2019 Nov 1;31(13):917-932. doi: 10.1089/ars.2018.7664. Epub 2019 Mar 20. Antioxid Redox Signal. 2019. PMID: 30652485 Free PMC article. - E3 ubiquitin ligase CHIP facilitates Toll-like receptor signaling by recruiting and polyubiquitinating Src and atypical PKC{zeta}.
Yang M, Wang C, Zhu X, Tang S, Shi L, Cao X, Chen T. Yang M, et al. J Exp Med. 2011 Sep 26;208(10):2099-112. doi: 10.1084/jem.20102667. Epub 2011 Sep 12. J Exp Med. 2011. PMID: 21911421 Free PMC article.
References
- Medzhitov R, Janeway C., Jr. N. Engl. J. Med. 2000;343:338–344. - PubMed
- Martin MU, Wesche H. Biochim. Biophys. Acta. 2002;1592:265–280. - PubMed
- Iwasaki A, Medzhitov R. Nature Immunol. 2004;5:987–995. - PubMed
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Aleojos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Science. 1998;282:2085–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-059524/AI/NIAID NIH HHS/United States
- R01 AI044936/AI/NIAID NIH HHS/United States
- R01 AI044936-06/AI/NIAID NIH HHS/United States
- AI-057490/AI/NIAID NIH HHS/United States
- R01 AI059524/AI/NIAID NIH HHS/United States
- R01 AI057490-04/AI/NIAID NIH HHS/United States
- R01 AI057490/AI/NIAID NIH HHS/United States
- R01 AI059524-04/AI/NIAID NIH HHS/United States
- AI-44936/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous