Brain mechanisms supporting spatial discrimination of pain - PubMed (original) (raw)
Brain mechanisms supporting spatial discrimination of pain
Yoshitetsu Oshiro et al. J Neurosci. 2007.
Abstract
Pain is a uniquely individual experience that is heavily shaped by evaluation and judgments about afferent sensory information. In visual, auditory, and tactile sensory modalities, evaluation of afferent information engages brain regions outside of the primary sensory cortices. In contrast, evaluation of sensory features of noxious information has long been thought to be accomplished by the primary somatosensory cortex and other structures associated with the lateral pain system. Using functional magnetic resonance imaging and a delayed match-to-sample task, we show that the prefrontal cortex, anterior cingulate cortex, posterior parietal cortex, thalamus, and caudate are engaged during evaluation of the spatial locations of noxious stimuli. Thus, brain mechanisms supporting discrimination of sensory features of pain extend far beyond the somatosensory cortices and involve frontal regions traditionally associated with affective processing and the medial pain system. These frontoparietal interactions are similar to those involved in the processing of innocuous information and may be critically involved in placing afferent sensory information into a personal historical context.
Figures
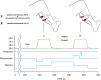
Figure 1.
The temporal sequence of the discrimination task. Noxious heat (or innocuous cool) stimuli were delivered sequentially to the back of the lower leg via two thermal probes separated by varying distances. Before the end of T2, subjects had to indicate whether T2 was in a different (or same) spatial location as T1. Discrimination-related brain activity was identified using regressors determined by response latencies in each individual discrimination trial. Additional regressors were used to identify brain activation that was related to spatial memory and to perceived pain (or cool).
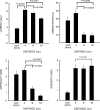
Figure 2.
Behavioral responses during spatial discrimination (means ± SEM). Discrimination of noxious heat became progressively more difficult with increasing probe proximity (16, 8, 4 cm). Response latencies, error rates, and subjective ratings of perceived difficulty increased monotonically as stimuli were delivered closer together. In contrast, perceived pain intensity exhibited a trend toward decreasing with increasing stimulus proximity. At 4 cm separation distances, cool discrimination was performed more rapidly than noxious heat discrimination but was nearly equally difficult. VAS, Visual analog scale.
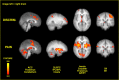
Figure 3.
Brain activation related to spatial discrimination of noxious stimuli is distinct from that related to perceived pain. These images are located at x = 0 mm, x = 30 mm, z = 5 mm, and y = −30 mm in standard stereotaxic space. IPL/SPL, Inferior parietal lobule/superior parietal lobule; GP/PT, globus pallidus/putamen; M1, primary motor cortex; DISCRIM., discrimination.
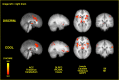
Figure 4.
Brain activation during spatial discrimination of innocuous cool stimuli. These images are located at x = 0 mm, x = 30 mm, z = 5 mm, and y = −30 mm in standard stereotaxic space. IPL/SPL, Inferior parietal lobule/superior parietal lobule; GP/PT, globus pallidus/putamen; M1, primary motor cortex, DISCRIM., discrimination.
Figure 5.
Brain activation during the memory period is related to the use of verbal mnemonic strategies. In >80% of discrimination trials, subjects used verbal strategies to remember the spatial location of the T1 stimulus. Consistent with such a verbal strategy, memory-related brain activation was lateralized to the left hemisphere in regions involved in verbal processing such as Broca's area on the inferior frontal gyrus. Activation during the memory period overlapped with activation during the discrimination period only in areas associated with motor planning. The left side of each brain image corresponds to the right side of the brain. IPL, Inferior parietal lobule; M1, primary motor cortex; DISCRIM., discrimination.
Similar articles
- Functional imaging of brain responses to pain. A review and meta-analysis (2000).
Peyron R, Laurent B, García-Larrea L. Peyron R, et al. Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review. - Functional activity mapping of the mesial hemispheric wall during anticipation of pain.
Porro CA, Cettolo V, Francescato MP, Baraldi P. Porro CA, et al. Neuroimage. 2003 Aug;19(4):1738-47. doi: 10.1016/s1053-8119(03)00184-8. Neuroimage. 2003. PMID: 12948728 - Functional MR imaging of regional brain activation associated with the affective experience of pain.
Fulbright RK, Troche CJ, Skudlarski P, Gore JC, Wexler BE. Fulbright RK, et al. AJR Am J Roentgenol. 2001 Nov;177(5):1205-10. doi: 10.2214/ajr.177.5.1771205. AJR Am J Roentgenol. 2001. PMID: 11641204 - Neural correlates of spatial working memory in humans: a functional magnetic resonance imaging study comparing visual and tactile processes.
Ricciardi E, Bonino D, Gentili C, Sani L, Pietrini P, Vecchi T. Ricciardi E, et al. Neuroscience. 2006 Apr 28;139(1):339-49. doi: 10.1016/j.neuroscience.2005.08.045. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16324793 - Pain and functional imaging.
Ingvar M. Ingvar M. Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1347-58. doi: 10.1098/rstb.1999.0483. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10466155 Free PMC article. Review.
Cited by
- Radiation of pain: Psychophysical evidence for a population coding mechanism in humans.
Adamczyk WM, Ramu V, Jackson C, Schulze G, Goldschneider KR, Kashikar-Zuck S, King CD, Coghill RC. Adamczyk WM, et al. bioRxiv [Preprint]. 2024 Aug 29:2024.04.02.587666. doi: 10.1101/2024.04.02.587666. bioRxiv. 2024. PMID: 38617343 Free PMC article. Preprint. - Parahippocampus hypertrophy drives gray matter morphological alterations in migraine patients without aura.
Yin T, Lan L, Tian Z, Li Z, Liu M, Gao Y, Liang F, Zeng F. Yin T, et al. J Headache Pain. 2023 May 17;24(1):53. doi: 10.1186/s10194-023-01588-z. J Headache Pain. 2023. PMID: 37193957 Free PMC article. - Spatial Tuning in Nociceptive Processing Is Driven by Attention.
Adamczyk WM, Katra M, Szikszay TM, Peugh J, King CD, Luedtke K, Coghill RC. Adamczyk WM, et al. J Pain. 2023 Jun;24(6):1116-1125. doi: 10.1016/j.jpain.2023.03.005. Epub 2023 Mar 23. J Pain. 2023. PMID: 36965648 Free PMC article. - Comorbid depressive symptoms can aggravate the functional changes of the pain matrix in patients with chronic back pain: A resting-state fMRI study.
Zhang G, Ma J, Lu W, Zhan H, Zhang X, Wang K, Hu Y, Wang X, Peng W, Yue S, Cai Q, Liang W, Wu W. Zhang G, et al. Front Aging Neurosci. 2022 Jul 18;14:935242. doi: 10.3389/fnagi.2022.935242. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35923542 Free PMC article. - Graph theory analysis identified two hubs that connect sensorimotor and cognitive and cortical and subcortical nociceptive networks in the non-human primate.
Wu R, Wang F, Yang PF, Gore JC, Chen LM. Wu R, et al. Neuroimage. 2022 Aug 15;257:119244. doi: 10.1016/j.neuroimage.2022.119244. Epub 2022 May 6. Neuroimage. 2022. PMID: 35533827 Free PMC article.
References
- Anourova I, Nikouline VV, Ilmoniemi RJ, Hotta J, Aronen HJ, Carlson S. Evidence for dissociation of spatial and nonspatial auditory information processing. NeuroImage. 2001;14:1268–1277. - PubMed
- Barrett DJ, Hall DA. Response preferences for “what” and “where” in human non-primary auditory cortex. NeuroImage. 2006;32:968–977. - PubMed
- Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. NeuroImage. 2004;23:224–232. - PubMed
- Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci. 1999;2:759–766. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical