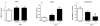1-methyl-4-phenylpyridinium-induced alterations of glutathione status in immortalized rat dopaminergic neurons - PubMed (original) (raw)
1-methyl-4-phenylpyridinium-induced alterations of glutathione status in immortalized rat dopaminergic neurons
Derek A Drechsel et al. Toxicol Appl Pharmacol. 2007.
Abstract
Decreased glutathione levels associated with increased oxidative stress are a hallmark of numerous neurodegenerative diseases, including Parkinson's disease. GSH is an important molecule that serves as an anti-oxidant and is also a major determinant of cellular redox environment. Previous studies have demonstrated that neurotoxins can cause changes in reduced and oxidized GSH levels; however, information regarding steady state levels remains unexplored. The goal of this study was to characterize changes in cellular GSH levels and its regulatory enzymes in a dopaminergic cell line (N27) following treatment with the Parkinsonian toxin, 1-methyl-4-phenylpyridinium (MPP(+)). Cellular GSH levels were initially significantly decreased 12 h after treatment, but subsequently recovered to values greater than controls by 24 h. However, oxidized glutathione (GSSG) levels were increased 24 h following treatment, concomitant with a decrease in GSH/GSSG ratio prior to cell death. In accordance with these changes, ROS levels were also increased, confirming the presence of oxidative stress. Decreased enzymatic activities of glutathione reductase and glutamate-cysteine ligase by 20-25% were observed at early time points and partly account for changes in GSH levels after MPP(+) exposure. Additionally, glutathione peroxidase activity was increased 24 h following treatment. MPP(+) treatment was not associated with increased efflux of glutathione to the medium. These data further elucidate the mechanisms underlying GSH depletion in response to the Parkinsonian toxin, MPP(+).
Figures
Figure 1
Effect of MPP+ on N27 cell viability after 24 (■) and 48 (▲) h incubations. Data presented as percentage of media LDH activity compared to total (media + cell lysate) LDH activity versus the log of MPP+ concentration (M). Data expressed as mean ± SE. *p < 0.05 versus time-matched controls, one-way ANOVA, n=6 per group.
Figure 2
Cellular GSH (A), GSSG (B) and GSH/GSSG (C) levels in N27 cells following MPP+ treatment for 24 h. Bars represent mean (% control) + SE. Bars with different letters were statistically different form one another (p < 0.05, one-way ANOVA, n=6 per group.
Figure 3
Cellular GSH levels in N27 cells following MPP+ treatment for 6, 12, or 24 h. Data points for control (■), 100 μM MPP+ (▲), and 1 mM MPP+ (▼) represent mean (% control) ± SE. *p < 0.05 versus controls, two-way ANOVA with Bonferroni post-test, n=6-7 per group.
Figure 4
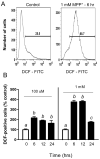
Intracellular ROS levels measured by DCF fluorescence via flow cytometry in N27 cells following MPP+ treatment. (A) Representative histograms of DCF fluorescence in vehicle- and 1 mM MPP+-treated N27 cells at 6 h. Numbers represent the percentage of cells with DCF positive staining compared to unlabeled controls. (B) ROS levels in N27 cells following treatment with 100 μM or 1 mM MPP+ for 6, 12, and 24 h. Data expressed as percentage of cells staining positive for DCF compared to controls. Bars represent mean (% control) + SE. Bars with different letters were statistically different from one another (p < 0.05, one-way ANOVA, n=3 per group.
Figure 5
Glutathione reductase (GR, A) and glutathione peroxidase (GPx, B) enzyme activities in N27 cells following MPP+ treatment for 6,12, and 24 h. Bars represent mean (% control) + SE. Bars with different letters were statistically different from one another (p < 0.05, one-way ANOVA, n=3 per group.
Figure 6
Glutamate-cysteine ligase (GCL, A) and glutathione synthetase (GS, B) enzyme activities in N27 cells following MPP+ treatment for 6, 12, and 24 h. Bars represent mean (% control) + SE. Bars with different letters were statistically different from one another (p < 0.05, one-way ANOVA, n=3 per group.
Similar articles
- Mangiferin protects against 1-methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A cells.
Amazzal L, Lapôtre A, Quignon F, Bagrel D. Amazzal L, et al. Neurosci Lett. 2007 May 17;418(2):159-64. doi: 10.1016/j.neulet.2007.03.025. Epub 2007 Mar 14. Neurosci Lett. 2007. PMID: 17433543 - Fibroblast growth factor 9 upregulates heme oxygenase-1 and gamma-glutamylcysteine synthetase expression to protect neurons from 1-methyl-4-phenylpyridinium toxicity.
Huang JY, Chuang JI. Huang JY, et al. Free Radic Biol Med. 2010 Sep 15;49(6):1099-108. doi: 10.1016/j.freeradbiomed.2010.06.026. Epub 2010 Jul 13. Free Radic Biol Med. 2010. PMID: 20615462 - [The different aspects of the biological role of glutathione].
Bilska A, Kryczyk A, Włodek L. Bilska A, et al. Postepy Hig Med Dosw (Online). 2007 Jul 11;61:438-53. Postepy Hig Med Dosw (Online). 2007. PMID: 17679914 Review. Polish. - Pro-oxidant shift in glutathione redox state during aging.
Rebrin I, Sohal RS. Rebrin I, et al. Adv Drug Deliv Rev. 2008 Oct-Nov;60(13-14):1545-52. doi: 10.1016/j.addr.2008.06.001. Epub 2008 Jul 4. Adv Drug Deliv Rev. 2008. PMID: 18652861 Free PMC article. Review.
Cited by
- Licofelone attenuates MPTP-induced neuronal toxicity: behavioral, biochemical and cellular evidence.
Gupta A, Kumar A, Kulkarni SK. Gupta A, et al. Inflammopharmacology. 2010 Oct;18(5):223-32. doi: 10.1007/s10787-010-0052-6. Epub 2010 Aug 11. Inflammopharmacology. 2010. PMID: 20697819 - Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons.
Braidy N, Selvaraju S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, Al-Senawi H, Abd Alrahman Alobaidy A, Lakhtakia R, Guillemin GJ. Braidy N, et al. Oxid Med Cell Longev. 2013;2013:685909. doi: 10.1155/2013/685909. Epub 2013 Oct 3. Oxid Med Cell Longev. 2013. PMID: 24223235 Free PMC article. - Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity.
Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Levesque S, et al. Brain. 2010 Mar;133(Pt 3):808-21. doi: 10.1093/brain/awp333. Epub 2010 Jan 31. Brain. 2010. PMID: 20123724 Free PMC article. - Post-translational Activation of Glutamate Cysteine Ligase with Dimercaprol: A NOVEL MECHANISM OF INHIBITING NEUROINFLAMMATION IN VITRO.
McElroy PB, Sri Hari A, Day BJ, Patel M. McElroy PB, et al. J Biol Chem. 2017 Mar 31;292(13):5532-5545. doi: 10.1074/jbc.M116.723700. Epub 2017 Feb 15. J Biol Chem. 2017. PMID: 28202547 Free PMC article. - MPP+-Lesioned Mice: an Experimental Model of Motor, Emotional, Memory/Learning, and Striatal Neurochemical Dysfunctions.
Cunha MP, Pazini FL, Lieberknecht V, Budni J, Oliveira Á, Rosa JM, Mancini G, Mazzardo L, Colla AR, Leite MC, Santos ARS, Martins DF, de Bem AF, Gonçalves CAS, Farina M, Rodrigues ALS. Cunha MP, et al. Mol Neurobiol. 2017 Oct;54(8):6356-6377. doi: 10.1007/s12035-016-0147-1. Epub 2016 Oct 8. Mol Neurobiol. 2017. PMID: 27722926
References
- Bahat-Stroomza M, Gilgun-Sherki Y, Offen D, Panet H, Saada A, Krool-Galron N, Barzilai A, Atlas D, Melamed E. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson's disease. Eur J Neurosci. 2005;21:637–646. - PubMed
- Bharath S, Andersen JK. Glutathione depletion in a midbrain-derived immortalized dopaminergic cell line results in limited tyrosine nitration of mitochondrial complex I subunits: implications for Parkinson's disease. Antioxid Redox Signal. 2005;7:900–910. - PubMed
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64:1037–1048. - PubMed
- Buhmann C, Arlt S, Kontush A, Moller-Bertram T, Sperber S, Oechsner M, Stuerenburg HJ, Beisiegel U. Plasma and CSF markers of oxidative stress are increased in Parkinson's disease and influenced by antiparkinsonian medication. Neurobiol Dis. 2004;15:160–170. - PubMed
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources