Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions - PubMed (original) (raw)
Review
Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions
Patrik Vuilleumier et al. Philos Trans R Soc Lond B Biol Sci. 2007.
Abstract
Visual processing is not determined solely by retinal inputs. Attentional modulation can arise when the internal attentional state (current task) of the observer alters visual processing of the same stimuli. This can influence visual cortex, boosting neural responses to an attended stimulus. Emotional modulation can also arise, when affective properties (emotional significance) of stimuli, rather than their strictly visual properties, influence processing. This too can boost responses in visual cortex, as for fear-associated stimuli. Both attentional and emotional modulation of visual processing may reflect distant influences upon visual cortex, exerted by brain structures outside the visual system per se. Hence, these modulations may provide windows onto causal interactions between distant but interconnected brain regions. We review recent evidence, noting both similarities and differences between attentional and emotional modulation. Both can affect visual cortex, but can reflect influences from different regions, such as fronto-parietal circuits versus the amygdala. Recent work on this has developed new approaches for studying causal influences between human brain regions that may be useful in other cognitive domains. The new methods include application of functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) measures in brain-damaged patients to study distant functional impacts of their focal lesions, and use of transcranial magnetic stimulation concurrently with fMRI or EEG in the normal brain. Cognitive neuroscience is now moving beyond considering the putative functions of particular brain regions, as if each operated in isolation, to consider, instead, how distinct brain regions (such as visual cortex, parietal or frontal regions, or amygdala) may mutually influence each other in a causal manner.
Figures
Figure 1
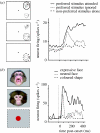
Single-cell recordings in the monkey illustrate two different types of modulation of visual responses. (a) Illustration of attentional effects for neurons in inferior temporal visual cortex (adapted from Chelazzi et al. 1998). When the ‘preferred’ stimulus is presented, responses are enhanced and prolonged if attention is directed to the stimulus as task-relevant (red), but weaker and more transient if attention is directed to another stimulus instead, with the preferred stimulus now being task-irrelevant (green). In this case, later components of the response can then be similar to when the preferred stimulus is absent (blue). (b) Illustration of emotional effects on face-selective neurons in superior temporal sulcus (adapted from Sugase et al. 1999). The pattern and time-course of emotional effects appear rather analogous to those of attention, with enhanced and prolonged responses when the seen face has an emotional expression (red), but weaker with a more transient peak when the seen face is neutral (green).
Figure 2
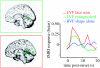
Example of fMRI results showing modulation of fusiform cortex responses to faces by spatial attention in the human brain (adapted from Vuilleumier et al. 2001_a_). Subjects saw displays that always contained a pair of faces (aligned either vertically or horizontally), together with a pair of houses, but concentrated on one pair only to perform a picture-matching task. The lateral face-selective fusiform area (FFA) was more strongly activated when faces appeared at the task-relevant location (red), while responses were strongly reduced when faces were task-irrelevant (green), although the visual displays were physically comparable in both conditions (and equated by counterbalancing). In comparison, FFA was not responsive to pictures of houses (blue), obtained in a separate localizer fMRI scan. Units for fMRI activation (betas) correspond to parameters estimates for event-related changes in BOLD signal.
Figure 3
Examples showing activation of attentional-control networks within frontal and parietal cortex in two different visual tasks. (a) Blood oxygen level-dependent (BOLD) fMRI activity is increased in bilateral intraparietal sulcus (IPS), middle frontal gyrus (possibly corresponding to the frontal eye field, FEF) and anterior cingulate cortex (ACC), when subjects have to focus their attention at fixation for a difficult visual task (rapid sequential visual presentation of targets among a stream of distractors, with high attention load), relative to an easy task at fixation with the same stimuli (low attentional load). Adapted from Schwartz et al. (2005). (b) A similar and overlapping network is activated during a visual search task, with increased activation when subjects have to detect a novel target (with different colour and different location) relative to a repeated target (with same colour but different location). Adapted from Kristjansson et al. (2006).
Figure 4
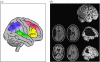
Common lesion sites in patients with unilateral spatial neglect. (a) Damage may involve different regions in both parietal and frontal lobes, most often the inferior parietal lobule (IPL) and temporo-parietal junction (TPJ), but also the middle frontal gyrus (MFG) and inferior frontal gyrus (IFG) and possibly the superior temporal gyrus (STG). These regions overlap with many of those areas associated with attentional control by fMRI studies in normals (cf. figure 3). (b) Lesions may have very different extents in different neglect patients, as shown here for two example cases (adapted from Driver & Vuilleumier 2001), often involving more than just one brain area within the attentional network.
Figure 5
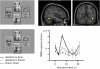
Effects of right parietal damage on visual cortex activation in a patient with left spatial neglect and visual extinction. Face stimuli were presented in the contralesional left hemifield with another distractor shape in the ipsilesional right hemifield, such that the faces were either perceived on some trials or extinguished from awareness on other trials (adapted from Vuilleumier et al. 2001_a_). When perceived, contralesional faces evoked significant BOLD fMRI responses in intact right occipital and temporal visual areas (red, here for a region of inferior temporal cortex posterior to FFA). Residual activation was still observed when contralesional faces were extinguished (green), relative to when there was no stimulus in the contralesional field (blue), but such activation was reduced relative to perceived faces (red). These data indicate that parietal damage may have significant functional consequences on the activation of intact visual areas in relation to conscious perception. Units for fMRI activation (betas) correspond to parameters estimates for event-related changes in BOLD signal.
Figure 6
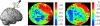
Illustration of concurrent TMS–fMRI study by Ruff et al. (2006), with example results from retinotopic visual cortex. TMS was applied over human right frontal-eye fields (see cartoon at left, depicting TMS stimulator held over this brain region). TMS stimulation was applied inside the MR scanner, interleaved with MR slice-acquisition, with procedures to prevent MR artefacts. Example fMRI results are shown for two participants, in ‘flat-map’ depictions of retinotopically mapped visual cortical areas. Borders between adjacent areas (e.g. V1 with ventral V2 (‘V2v’), or with dorsal V2 (‘V2d’) and so on) are drawn in black, with areas labelled. Foveal confluence is marked with a cross, with increased retinal eccentricity running out from here within each marked visual area. The ‘hot’ colours correspond to increased fMRI activity with higher TMS intensity to FEF; ‘cold’ colours represent decreased activity instead. The consistent pattern in all subjects was that, for all retinotopic areas (V1–V4), representations of the peripheral visual field showed enhanced fMRI activity with increased FEF-TMS intensity, while the central visual field (nearer the foveal cross) showed reduced activity. This confirms that human FEF can causally modulate activity in retinotopic visual cortex. Ruff et al. (2006) also derived and confirmed the psychophysical prediction, based on these fMRI data, that TMS should enhance peripheral relative to central vision, for perceived contrast.
Figure 7
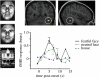
Example of emotional modulation of activation in FFA. Results from fMRI in normal subjects show that FFA exhibits selective responses to pictures of faces (green), not to houses (blue), but also exhibits a further increase to emotionally salient facial expression, particularly when fearful or threat-related (red). Data from P. Vuilleumier & G. Pourtois (2005, unpublished); see also Vuilleumier et al. 2001_a_). Units for fMRI activation (betas) correspond to parameters estimates for event-related changes in BOLD signal.
Figure 8
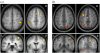
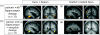
Effect of amygdala lesion on fMRI BOLD responses to emotional faces in visual cortex (adapted from Vuilleumier et al. 2004_a_). Patients with medial temporal-lobe sclerosis, whose lesions involved either (a) the hippocampus alone or (b) the hippocampus plus amygdala, performed a picture-matching task similar to that illustrated in figure 2. Seen faces could be fearful or neutral and task-relevant or not. Patients with hippocampal damage alone (a) showed normal activation of fusiform cortex for fearful versus neutral faces (a(ii)), as for healthy subjects also (not shown). Patients with additional damage to the amygdala (b) showed no effect of fearful expression in visual cortex. By contrast, in both patient groups, fusiform cortex was normally activated by attention to task-relevant faces (a(i), b(i)). These results indicate distant functional consequences for visual cortex responses to fearful faces, caused by amygdala damage.
Similar articles
- Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging.
Vuilleumier P, Pourtois G. Vuilleumier P, et al. Neuropsychologia. 2007 Jan 7;45(1):174-94. doi: 10.1016/j.neuropsychologia.2006.06.003. Epub 2006 Jul 18. Neuropsychologia. 2007. PMID: 16854439 Review. - Functional MRI and EEG Index Complementary Attentional Modulations.
Itthipuripat S, Sprague TC, Serences JT. Itthipuripat S, et al. J Neurosci. 2019 Jul 31;39(31):6162-6179. doi: 10.1523/JNEUROSCI.2519-18.2019. Epub 2019 May 24. J Neurosci. 2019. PMID: 31127004 Free PMC article. - Brain activity underlying visual perception and attention as inferred from TMS-EEG: a review.
Taylor PC, Thut G. Taylor PC, et al. Brain Stimul. 2012 Apr;5(2):124-9. doi: 10.1016/j.brs.2012.03.003. Epub 2012 Mar 25. Brain Stimul. 2012. PMID: 22494831 Review. - Anticipatory alpha phase influences visual working memory performance.
Zanto TP, Chadick JZ, Gazzaley A. Zanto TP, et al. Neuroimage. 2014 Jan 15;85 Pt 2(0 2):794-802. doi: 10.1016/j.neuroimage.2013.07.048. Epub 2013 Jul 25. Neuroimage. 2014. PMID: 23891902 Free PMC article. - Concurrent TMS-fMRI Reveals Interactions between Dorsal and Ventral Attentional Systems.
Leitão J, Thielscher A, Tünnerhoff J, Noppeney U. Leitão J, et al. J Neurosci. 2015 Aug 12;35(32):11445-57. doi: 10.1523/JNEUROSCI.0939-15.2015. J Neurosci. 2015. PMID: 26269649 Free PMC article.
Cited by
- Statistical learning as a tool for rehabilitation in spatial neglect.
Shaqiri A, Anderson B, Danckert J. Shaqiri A, et al. Front Hum Neurosci. 2013 May 29;7:224. doi: 10.3389/fnhum.2013.00224. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23754998 Free PMC article. - Emotionally anesthetized: media violence induces neural changes during emotional face processing.
Stockdale LA, Morrison RG, Kmiecik MJ, Garbarino J, Silton RL. Stockdale LA, et al. Soc Cogn Affect Neurosci. 2015 Oct;10(10):1373-82. doi: 10.1093/scan/nsv025. Epub 2015 Mar 9. Soc Cogn Affect Neurosci. 2015. PMID: 25759472 Free PMC article. - Neural correlates of opposing effects of emotional distraction on perception and episodic memory: an event-related FMRI investigation.
Shafer AT, Dolcos F. Shafer AT, et al. Front Integr Neurosci. 2012 Sep 19;6:70. doi: 10.3389/fnint.2012.00070. eCollection 2012. Front Integr Neurosci. 2012. PMID: 23049502 Free PMC article. - Processing of emotional words measured simultaneously with steady-state visually evoked potentials and near-infrared diffusing-wave spectroscopy.
Koban L, Ninck M, Li J, Gisler T, Kissler J. Koban L, et al. BMC Neurosci. 2010 Jul 27;11:85. doi: 10.1186/1471-2202-11-85. BMC Neurosci. 2010. PMID: 20663220 Free PMC article.
References
- Amaral D.G, Price J.L. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi:10.1002/cne.902300402 - DOI - PubMed
- Amaral D.G, Behniea H, Kelly J.L. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi:10.1016/S0306-4522(02)01001-1 - DOI - PubMed
- Anderson A.K. Affective influences on the attentional dynamics supporting awareness. J. Exp. Psychol. Gen. 2005;134:258–281. doi:10.1037/0096-3445.134.2.258 - DOI - PubMed
- Anderson A.K, Phelps E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi:10.1038/35077083 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources