From cognitive to neural models of working memory - PubMed (original) (raw)
Review
From cognitive to neural models of working memory
Mark D'Esposito. Philos Trans R Soc Lond B Biol Sci. 2007.
Abstract
Working memory refers to the temporary retention of information that was just experienced or just retrieved from long-term memory but no longer exists in the external environment. These internal representations are short-lived, but can be stored for longer periods of time through active maintenance or rehearsal strategies, and can be subjected to various operations that manipulate the information in such a way that makes it useful for goal-directed behaviour. Empirical studies of working memory using neuroscientific techniques, such as neuronal recordings in monkeys or functional neuroimaging in humans, have advanced our knowledge of the underlying neural mechanisms of working memory. This rich dataset can be reconciled with behavioural findings derived from investigating the cognitive mechanisms underlying working memory. In this paper, I review the progress that has been made towards this effort by illustrating how investigations of the neural mechanisms underlying working memory can be influenced by cognitive models and, in turn, how cognitive models can be shaped and modified by neuroscientific data. One conclusion that arises from this research is that working memory can be viewed as neither a unitary nor a dedicated system. A network of brain regions, including the prefrontal cortex (PFC), is critical for the active maintenance of internal representations that are necessary for goal-directed behaviour. Thus, working memory is not localized to a single brain region but probably is an emergent property of the functional interactions between the PFC and the rest of the brain.
Figures
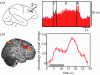
Figure 1
Neural activity in the monkey and the human lateral PFC during the retention interval of a spatial oculomotor delayed response (ODR) task. (a) Macaque: average of single-unit recordings from 46 neurons with delay-period activity from the monkey lateral PFC (brain area (BA) area 46; adapted from Funahashi et al. 1989). C, cue; D, delay; R, response. (b) Human: significant delay-period activity (left) and average (±s.e.) fMRI signal (right) from right lateral PFC (BA area 46; circled) in a human performing an ODR task (unpublished data from my laboratory). The grey bar represents the length of the delay interval. Note that how in both cases the level of PFC activity persists throughout the delay, seconds after the stimulus cue has disappeared.
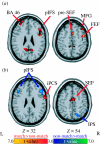
Figure 2
Statistical parametric _t_-maps contrasting oculomotor delayed matching-to-sample versus non-matching-to-sample delay period-specific activity (Curtis et al. 2004). Activity during the (a) early and (b) late delay periods is shown. Warm colours depict regions with greater delay-period activity on matching than non-matching trials. Cool colours depict regions with greater delay-period activity on non-matching than matching trials. BA, brain area; FEF, frontal eye fields; SEF, supplementary eye fields; MFG, middle frontal gyrus; pIFS, posterior inferior frontal sulcus; iPCS, inferior precentral sulcus; IPS, intraparietal sulcus.
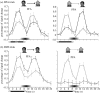
Figure 3
Human inferior temporal cortex activity during visual working memory maintenance and associative memory retrieval (Ranganath et al. 2004). (a) DPA trials: on DPA trials, activity during the cue phase in the FFA (left) and PPA (right) was enhanced when each region's preferred stimulus was presented (black line, face stimuli; grey line, house stimuli). However, during the delay period, activity in these regions reflected the type of information that was active in memory, rather than the previously presented cue stimulus, i.e. delay activity in the FFA was greater when a face was recalled in response to a house cue and delay activity in the PPA was greater when a house was recalled in response to a face cue. (b) DMS trials: on DMS trials, cue and delay-period activity in the FFA and PPA was enhanced when subjects maintained each region's preferred stimulus type (black dashed line, face stimuli; grey dashed line, house stimuli).
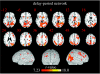
Figure 4
Delay-period correlation map with right FFA seed (_N_=17; Gazzaley et al. 2004). Activations are thresholded at p<0.05 (corrected) and shown overlaid on both axial slices and a three-dimensionally rendered MNI template brain. The colour scale indicates the magnitude of the _t_-values.
Figure 5
(a) fMRI and (b) ERP data during the performance of a face/scene delay task in healthy human individuals (Gazzaley et al. 2005_a_). The left-hand graph shows fMRI signal from the right FFA during the three behavioural conditions. fMRI signal is greatest during the ‘remember faces’ condition and least during the ‘ignore faces’ condition. The right-hand graphs show the average N170 peak latency values during the three behavioural conditions. N170 latency is earliest during the ‘remember faces’ condition and latest during the ‘ignore faces’ condition.
Similar articles
- Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies.
D'Esposito M, Postle BR, Rypma B. D'Esposito M, et al. Exp Brain Res. 2000 Jul;133(1):3-11. doi: 10.1007/s002210000395. Exp Brain Res. 2000. PMID: 10933205 Review. - The functional neuroanatomy of working memory: contributions of human brain lesion studies.
Müller NG, Knight RT. Müller NG, et al. Neuroscience. 2006 Apr 28;139(1):51-8. doi: 10.1016/j.neuroscience.2005.09.018. Epub 2005 Dec 15. Neuroscience. 2006. PMID: 16352402 Review. - Stable population coding for working memory coexists with heterogeneous neural dynamics in prefrontal cortex.
Murray JD, Bernacchia A, Roy NA, Constantinidis C, Romo R, Wang XJ. Murray JD, et al. Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):394-399. doi: 10.1073/pnas.1619449114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028221 Free PMC article. - Working memory capacity of crows and monkeys arises from similar neuronal computations.
Hahn LA, Balakhonov D, Fongaro E, Nieder A, Rose J. Hahn LA, et al. Elife. 2021 Dec 3;10:e72783. doi: 10.7554/eLife.72783. Elife. 2021. PMID: 34859781 Free PMC article. - A large-scale neurocomputational model of task-oriented behavior selection and working memory in prefrontal cortex.
Chadderdon GL, Sporns O. Chadderdon GL, et al. J Cogn Neurosci. 2006 Feb;18(2):242-57. doi: 10.1162/089892906775783624. J Cogn Neurosci. 2006. PMID: 16494684
Cited by
- Verbal and Spatial Working Memory Capacity in Blind Adults and the Possible Influence of Age at Blindness Onset: A Systematic Review and Meta-analysis.
Sepúlveda-Palomo M, Del Río D, Villalobos D, Fernández González S. Sepúlveda-Palomo M, et al. Neuropsychol Rev. 2024 Oct 14. doi: 10.1007/s11065-024-09651-5. Online ahead of print. Neuropsychol Rev. 2024. PMID: 39397144 Review. - Spatial Orientation Assessment in the Elderly: A Comprehensive Review of Current Tests.
Tragantzopoulou P, Giannouli V. Tragantzopoulou P, et al. Brain Sci. 2024 Sep 5;14(9):898. doi: 10.3390/brainsci14090898. Brain Sci. 2024. PMID: 39335393 Free PMC article. Review. - Maintenance and transformation of representational formats during working memory prioritization.
Pacheco-Estefan D, Fellner MC, Kunz L, Zhang H, Reinacher P, Roy C, Brandt A, Schulze-Bonhage A, Yang L, Wang S, Liu J, Xue G, Axmacher N. Pacheco-Estefan D, et al. Nat Commun. 2024 Sep 19;15(1):8234. doi: 10.1038/s41467-024-52541-w. Nat Commun. 2024. PMID: 39300141 Free PMC article. - Inter-individual variability (but intra-individual stability) of overt versus covert rehearsal strategies in a digital Corsi task.
de Sardenberg Schmid L, Hardiess G. de Sardenberg Schmid L, et al. J Vis. 2024 Aug 1;24(8):2. doi: 10.1167/jov.24.8.2. J Vis. 2024. PMID: 39087936 Free PMC article. - Towards optimized methodological parameters for maximizing the behavioral effects of transcranial direct current stimulation.
Santander T, Leslie S, Li LJ, Skinner HE, Simonson JM, Sweeney P, Deen KP, Miller MB, Brunye TT. Santander T, et al. Front Hum Neurosci. 2024 Jul 2;18:1305446. doi: 10.3389/fnhum.2024.1305446. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39015825 Free PMC article.
References
- Anderson J.R.The architecture of cognition1983Harvard University Press; Cambridge, MA
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci. 2001;5:119–126. doi:10.1016/S1364-6613(00)01593-X - DOI - PubMed
- Awh E, Jonides J, Smith E.E, Schumacher E.H, Koeppe R.A, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from PET. Psychol. Sci. 1996;7:25–31. doi:10.1111/j.1467-9280.1996.tb00662.x - DOI
- Awh E, Jonides J, Smith E.E, Buxton R.B, Frank L.R, Love T, Wong E.C, Gmeindl L. Rehearsal in spatial working memory: evidence from neuroimaging. Psychol. Sci. 1999;10:433–437. doi:10.1111/1467-9280.00182 - DOI
- Baddeley A. Oxford University Press; New York, NY: 1986. Working memory.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous