Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells - PubMed (original) (raw)
Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells
Josephine Sui-Yan Au et al. J Cell Biol. 2007.
Abstract
In polarized epithelial cells, newly synthesized membrane proteins are delivered on specific pathways to either the apical or basolateral domains, depending on the sorting motifs present in these proteins. Because myosin VI has been shown to facilitate secretory traffic in nonpolarized cells, we investigated its role in biosynthetic trafficking pathways in polarized MDCK cells. We observed that a specific splice isoform of myosin VI with no insert in the tail domain is required for the polarized transport of tyrosine motif containing basolateral membrane proteins. Sorting of other basolateral or apical cargo, however, does not involve myosin VI. Site-directed mutagenesis indicates that a functional complex consisting of myosin VI, optineurin, and probably the GTPase Rab8 plays a role in the basolateral delivery of membrane proteins, whose sorting is mediated by the clathrin adaptor protein complex (AP) AP-1B. Our results suggest that myosin VI is a crucial component in the AP-1B-dependent biosynthetic sorting pathway to the basolateral surface in polarized epithelial cells.
Figures
Figure 1.
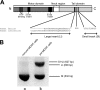
Expression of myosin VI isoforms in MDCK cells. (A) Schematic diagram of myosin VI domain organization. The motor domain contains two unique inserts; the 23-aa insert modulates its ATPase activity and the 53-aa insert is the “reverse gear” that controls its directionality along actin filaments. The tail domain is alternatively spliced with two insertions, a LI at the end of the helical domain and a SI within the globular domain. A long version (31 aa) of the LI isoform is expressed in MDCK cells, whereas the SI sequence is conserved between MDCK cells and human brain. (B) Expression of myosin VI isoforms in nonpolarized and polarized MDCK cells. Nonpolarized MDCK cells mainly express the myosin VI isoform with NI (a), whereas MDCK cells grown on filters for 4 d express three different isoforms with NI, the LI, and the SI+LI (b).
Figure 2.
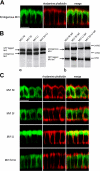
Localization of myosin VI isoforms in polarized MDCK cells. (A) Endogenous myosin VI and actin are found at the apical, as well as the basolateral, domain in wild-type MDCK cells. In B, the expression levels of GFP-myosin VI isoforms and GFP-myosin VI tail isoforms are compared by Western blotting to the amount of endogenous myosin VI expressed in stable MDCK cell lines. Note the expression levels of all the expressed GFP-myosin VI and GFP-tails are roughly equal to the amount of endogenous myosin VI. (C) Each myosin VI isoform exhibits a distinct, but overlapping, localization in MDCK cells. (left) Localization of GFP-myosin VI isoforms in MDCK stable cell lines. (middle) Actin distribution stained with rhodamine phalloidin. (right) Merged image of GFP-myosin VI and actin. Bars, 10 μm.
Figure 3.
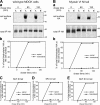
Expression of the myosin VI tail NI isoform missorts VSV-G to the apical domain in MDCK cells. (A) In wild-type MDCK cells, VSV-G is sorted and transported to the basolateral domain. Fully polarized MDCK cells were infected for 18 h with VSV-G, and the next day they were pulse labeled with [35S]methionine for 20 min before chasing for 30, 60, 90, 120, and 180 min. At each time point, the apical and the basolateral surfaces were biotinylated and the total VSV-G was immunoprecipitated. From this pool, the biotinylated VSV-G was precipitated using streptavidin beads. (a) The autoradiography of apical (A) VSV-G, basolateral (B) VSV-G, and total immunoprecipitated (IP) VSV-G in wild-type MDCK cells. (b) Graphical representation of the percentage of total biotinylated VSV-G reaching the apical (solid line) or the basolateral (dashed line) cell surface. (B) VSV-G is missorted to the apical surface in MDCK cells overexpressing the myosin VI NI tail. (C–E) VSV-G is sorted normally to the basolateral domain in MDCK cells overexpressing the myosin VI tail with SI, LI, or SI+LI.
Figure 4.
Myosin VI is not involved in sorting of HA to the apical domain in MDCK cells. (A) In wild-type MDCK cells, HA is sorted and transported to the apical domain. (a) The autoradiography of apical (A) HA, basolateral (B) HA, and total immunoprecipitated (IP) HA. (b) Graphical representation of the percentage of total biotinylated HA that has reached either the apical (solid line) or the basolateral (dashed line) cell surface. (B) HA is correctly sorted to the apical surface in MDCK cells overexpressing the myosin VI NI tail. C–E are also sorted normally to the apical domain in MDCK cells overexpressing the myosin VI tail with SI, LI, or SI+LI.
Figure 5.
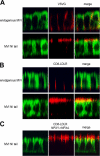
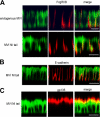
Expression of the myosin VI NI tail isoform results in missorting of AP-1B–dependent cargo in MDCK cells. In A and B, the immunofluorescent localization of VSV-G and CD8-LDLR in wild-type MDCK cells and MDCK cells expressing the myosin VI NI tail are shown. In C, the localization of CD8-LDLR containing a mutation in the NPXY endocytosis signal (NPXY–NPXA) in MDCK cells expressing myosin VI NI tail is shown. VSV-G and the LDLR are AP-1B–dependent cargos that are sorted to the basolateral domain in wild-type MDCK cells, but are localized at the apical domain in cells overexpressing the myosin VI NI tail. The endocytosis-defective CD8-LDLR is directly mistargeted to the apical domain, and no transcytosis step after endocytosis at the basolateral domain is involved. MDCK cells were infected with the virus encoding tsO45 VSV-G and incubated at 40°C overnight to accumulate VSV-G in the ER before a 2-h chase at 31°C in the presence of cycloheximide allowed the VSV-G to exit and accumulate on the cell surface. Cells infected with CD8-LDLR were incubated in the presence of cyclohexamide at 20°C for 3 h to accumulate the protein in the Golgi before release at 37°C. Cells were fixed without permeabilization and stained for cell surface VSV-G using an antibody to the luminal domain of VSV-G or with an antibody to CD8 to detect the CD8-LDLR chimera. The cells were permeabilized and stained for endogenous myosin VI in wild-type MDCK cells and for GFP in cells overexpressing myosin VI NI tail. Confocal vertical X–Z optical sections are shown. Bar, 10 μm.
Figure 6.
Expression of the myosin VI NI tail has no affect on FcγRIIB sorting or the localization of E-cadherin and gp135 in MDCK cells. FcγRIIB is sorted and transported correctly to the basolateral domain in both wild-type and myosin VI NI tail–expressing cells. (A, left) The localization of endogenous myosin VI in wild-type MDCK cells or in cells expressing GFP-myosin VI NI tail. (A, middle) The distribution of FcγRIIB in the cells. (A, right) Merged images. MDCK cells were infected overnight with virus encoding FcγRIIB, and then incubated at 20°C to accumulate the protein in the Golgi for 2 h, before a chase at 37°C in the presence of cycloheximide. Cells were fixed and stained for the FcγRIIB on the cell surface before permeabilization and staining for endogenous myosin VI in wild-type MDCK cells or for GFP in cells expressing GFP-myosin VI NI tail. MDCK cells expressing the myosin VI NI tail show normal steady-state distribution of the basolateral marker E-cadherin (B) and the apical marker protein gp135 (C). (left) The localization of the GFP-myosin VI NI tail. (middle) Transfected cells stained with antibodies either to E-cadherin or gp135. (right) Merged image of the corresponding left and middle images. Confocal vertical X–Z optical sections are shown. Bars, 10 μm.
Figure 7.
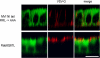
When the optineurin binding site in the myosin VI NI tail is mutated, VSV-G is not missorted to the apical domain. In MDCK cells expressing the myosin VI NI tail with the RRL–AAA mutation, VSV-G is sorted normally to the basolateral domain, whereas in the MDCK cells expressing the constitutively active GFP-Rab 8 Q67L, VSV-G is missorted to the apical domain. Stable MDCK cell lines expressing either mutant GFP-myosin VI NI tail, RRL–AAA or constitutively active GFP-Rab8-Q67L were grown on filters and fully polarized before infecting with the virus encoding tsO45 VSV-G. The infected cells were incubated at 40°C overnight to accumulate VSV-G in the ER, before a 2-h chase at 31°C in the presence of cycloheximide. Cells were fixed without permeabilization and stained for cell surface VSV-G using an antibody to the luminal domain of VSV-G. After permeabilization, GFP-tagged NI mutant tail or Rab8Q67L were detected with an antibody to GFP. (right) The merged image of the corresponding left and middle images. Confocal vertical X–Z optical sections are shown. Bar, 13 μm.
Figure 8.
Myosin VI and optineurin colocalize with AP-1, Rab8, and TfR in recycling endosomes. Cryosections of polarized MDCK cells were double labeled with antibodies against endogenous AP-1 (10-nm gold) and myosin VI (15-nm gold) or with antibodies against AP-1 (10-nm gold) and optineurin (15-nm gold). Cryosections of polarized MDCK cells stably expressing GFP-Rab8 were labeled with antibodies against endogenous myosin VI (15-nm gold) and with antibodies to GFP for localization of Rab8 (10-nm gold). For further characterization, the human TfR was expressed in MDCK cells and an antibody uptake experiment with a monoclonal antibody to the human TfR was performed. Cryosections of these cells show colocalization of endogenous myosin VI (10-nm gold) and TfR (15-nm gold) in recycling endosomes. Myosin VI and optineurin are present on AP-1–, Rab8-, and TfR-positive recycling endosomes and small clathrin–coated vesicles surrounding these endosomes. Arrowheads show colocalization of myosin VI or optineurin with AP-1, Rab8, or TfR. Bars, 450 nm.
Similar articles
- Myosin VI and optineurin are required for polarized EGFR delivery and directed migration.
Chibalina MV, Poliakov A, Kendrick-Jones J, Buss F. Chibalina MV, et al. Traffic. 2010 Oct;11(10):1290-303. doi: 10.1111/j.1600-0854.2010.01101.x. Traffic. 2010. PMID: 20604900 Free PMC article. - The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells.
Ang AL, Fölsch H, Koivisto UM, Pypaert M, Mellman I. Ang AL, et al. J Cell Biol. 2003 Oct 27;163(2):339-50. doi: 10.1083/jcb.200307046. J Cell Biol. 2003. PMID: 14581456 Free PMC article. - Analyzing the role of AP-1B in polarized sorting from recycling endosomes in epithelial cells.
Fölsch H. Fölsch H. Methods Cell Biol. 2015;130:289-305. doi: 10.1016/bs.mcb.2015.03.023. Epub 2015 Jun 11. Methods Cell Biol. 2015. PMID: 26360041 - Myosin VI and its cargo adaptors - linking endocytosis and autophagy.
Tumbarello DA, Kendrick-Jones J, Buss F. Tumbarello DA, et al. J Cell Sci. 2013 Jun 15;126(Pt 12):2561-70. doi: 10.1242/jcs.095554. Epub 2013 Jun 18. J Cell Sci. 2013. PMID: 23781020 Free PMC article. Review. - The building blocks for basolateral vesicles in polarized epithelial cells.
Fölsch H. Fölsch H. Trends Cell Biol. 2005 Apr;15(4):222-8. doi: 10.1016/j.tcb.2005.02.006. Trends Cell Biol. 2005. PMID: 15817379 Review.
Cited by
- Analysis of AQP4 trafficking vesicle dynamics using a high-content approach.
Mazzaferri J, Costantino S, Lefrancois S. Mazzaferri J, et al. Biophys J. 2013 Jul 16;105(2):328-37. doi: 10.1016/j.bpj.2013.06.010. Biophys J. 2013. PMID: 23870254 Free PMC article. - Myosin-X functions in polarized epithelial cells.
Liu KC, Jacobs DT, Dunn BD, Fanning AS, Cheney RE. Liu KC, et al. Mol Biol Cell. 2012 May;23(9):1675-87. doi: 10.1091/mbc.E11-04-0358. Epub 2012 Mar 14. Mol Biol Cell. 2012. PMID: 22419816 Free PMC article. - Myosin VI in skeletal muscle: its localization in the sarcoplasmic reticulum, neuromuscular junction and muscle nuclei.
Karolczak J, Sobczak M, Majewski L, Yeghiazaryan M, Jakubiec-Puka A, Ehler E, Sławińska U, Wilczyński GM, Rędowicz MJ. Karolczak J, et al. Histochem Cell Biol. 2013 Jun;139(6):873-85. doi: 10.1007/s00418-012-1070-9. Epub 2012 Dec 30. Histochem Cell Biol. 2013. PMID: 23275125 Free PMC article. - Rab8 interacts with distinct motifs in alpha2B- and beta2-adrenergic receptors and differentially modulates their transport.
Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H, Wu G. Dong C, et al. J Biol Chem. 2010 Jun 25;285(26):20369-80. doi: 10.1074/jbc.M109.081521. Epub 2010 Apr 27. J Biol Chem. 2010. PMID: 20424170 Free PMC article. - Myosins in cell junctions.
Liu KC, Cheney RE. Liu KC, et al. Bioarchitecture. 2012 Sep-Oct;2(5):158-70. doi: 10.4161/bioa.21791. Epub 2012 Sep 1. Bioarchitecture. 2012. PMID: 22954512 Free PMC article. Review.
References
- Buss, F., J. Kendrick-Jones, C. Lionne, A.E. Knight, G.P. Cote, and J. Paul Luzio. 1998. The localization of myosin VI at the Golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol. 143:1535–1545. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources