Thirty-one flavors of Drosophila rab proteins - PubMed (original) (raw)
Thirty-one flavors of Drosophila rab proteins
Jun Zhang et al. Genetics. 2007 Jun.
Abstract
Rab proteins are small GTPases that play important roles in transport of vesicle cargo and recruitment, association of motor and other proteins with vesicles, and docking and fusion of vesicles at defined locations. In vertebrates, >75 Rab genes have been identified, some of which have been intensively studied for their roles in endosome and synaptic vesicle trafficking. Recent studies of the functions of certain Rab proteins have revealed specific roles in mediating developmental signal transduction. We have begun a systematic genetic study of the 33 Rab genes in Drosophila. Most of the fly proteins are clearly related to specific vertebrate proteins. We report here the creation of a set of transgenic fly lines that allow spatially and temporally regulated expression of Drosophila Rab proteins. We generated fluorescent protein-tagged wild-type, dominant-negative, and constitutively active forms of 31 Drosophila Rab proteins. We describe Drosophila Rab expression patterns during embryogenesis, the subcellular localization of some Rab proteins, and comparisons of the localization of wild-type, dominant-negative, and constitutively active forms of selected Rab proteins. The high evolutionary conservation and low redundancy of Drosophila Rab proteins make these transgenic lines a useful tool kit for investigating Rab functions in vivo.
Figures
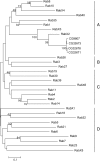
Figure 1.—
Phylogenetic tree of 33 predicted Drosophila Rab proteins. The number shown between each pair of branches is the bootstrap value that measures how consistent the data are. The value is calculated from a new data set (a pseudosample) by randomly copying one character from the original data matrix. It represents the percentage of 1000 bootstrap pseudosamples with replacement supporting that branch. Only bootstrap values >40% are shown. The length of the unit represents the divergence of proteins.
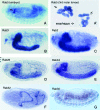
Figure 2.—
In situ hybridization of Drosophila Rab gene probes to localize transcripts in Drosophila whole-mount embryos. For each Rab gene, one stained preparation of a particular embryonic stage is shown. The stage that has the most representative staining pattern is shown. (A and A′) Rab5 mRNA signals in embryos (A) and third instar larvae (A′). (B–G) In situ patterns of Rab3, Rab2, Rab26, RabX4, Rab32, and Rab30.
Figure 3.—
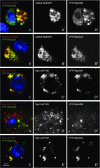
Conserved subcellular localization of mammalian and fly Rab proteins. The mammalian versions of well-characterized Rab5, Rab7, and Rab11 were cloned with a N-terminal YFP tag (shown in green and gray). Each pair of mammalian and dsRed-tagged Drosophila Rab proteins 9 (shown in red and gray) was cotransfected into HeLa cells and visualized with a Leica confocal microscope. Drosophila Rab5, Rab7, and Rab11 are localized in a punctate pattern and colocalized with mammalian Rab5 (A–A′′), Rab7 (B–B′′), and Rab11 (C–C′′), respectively. As a control, mammalian Rab5 and Drosophila Rab7 are not colocalized (D–D′′).
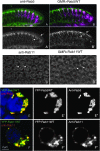
Figure 4.—
Colocalization of YFP-Rab5WT and YFP-Rab11WT with endogenous Rab5 and Rab11 proteins. (A and B) Longitudinal sections of third instar larval eye discs. Photoreceptor stainings with mAb 24B10 are in magenta. A and A′ show Rab5 antibody staining in green and gray, respectively, revealing a punctate localization that is enriched distally (between arrows). B and B′ show YFP-Rab5WT driven in photoreceptors with GMR-GAL4. The overexpressed fusion protein exhibits the same localization pattern as the endogenous protein. (C and D) Anti-Rab 11 staining (C) and photoreceptor-driven YFP-Rab11WT (D) in third instar eye imaginal discs. The cross sections reveal very similar localization patterns. Note the ring-like structure of Rab11-positive vesicles around the rhabdomeres (arrows). (E and E′′) Colocalization of endogenous Rab5 and exogenous tagged Rab5 proteins in S2R+ cells. Cells were transfected with YFP-Rab5WT and then fixed and stained with anti-Rab5 antibody (red and gray). (F and F′′) Colocalization of endogenous Rab11 and exogenous tagged Rab11 proteins in S2R+ cells. Cells were transfected with YFP-Rab11WT and then fixed and stained with anti-Rab11 antibody (red and gray).
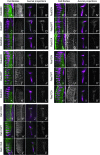
Figure 5.—
Comparison of tagged protein localization of WT, DN, and CA versions of Drosophila Rab proteins. YFP-Rab variants were expressed specifically in photoreceptors of transgenic flies using GMR-GAL4. Images of third instar eye-disc sections and terminal axonal projections of photoreceptors in the optic lobe are shown for Rab3, Rab4, Rab5, Rab7, and Rab11. Tissues were stained with the photoreceptor-specific antibody mAb 24B10 (magenta). YFP-Rab proteins are shown in green and gray.
Figure 6.—
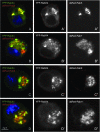
Reduction of YFP-Rab5DN endosomal localization. (A and B) dsRed-Rab5WT (red and gray) and YFP-Rab5DN (A; green and gray) or YFP-Rab5CA (B; green and gray) were coexpressed in cultured Drosophila S2R+ cells. dsRed-Rab5WT and YFP-Rab5CA are mainly colocalized while dsRed-Rab5WT and YFP-Rab5DN are not. (C–E) A pUAST-myc-2xFYVE (red and gray) construct that labels early endosomes was cotransfected with pUAST-YFP-Rab5WT (C), pUAST-YFP-Rab5DN (D), or pUAST-YFP-Rab5CA (E). YFP-Rab5WT and YFP-Rab5CA are colocalized mainly with Myc-2xFYVE whereas YFP-Rab5DN is not.
Figure 7.—
The subcellular localization of a newly studied type of Drosophila Rab, RabX4. (A) dsRed-tagged Rab5 (red and gray) and YFP-tagged RabX4 (green and grays) were cotransfected into Drosophila S2R+ cells. The majority of the RabX4 protein colocalized with Rab5, a protein reported to be located in the early endosome. (B–D) dsRed-tagged Rab7 (B), Rab9 (C), or Rab11(D) and YFP-tagged RabX4 were cotransfected into Drosophila S2R+ cells, respectively. No colocalization was observed between each pair of proteins.
Similar articles
- Systematic discovery of Rab GTPases with synaptic functions in Drosophila.
Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, Perkins TJ, Brankatschk M, Rothenfluh A, Buszczak M, Hiesinger PR. Chan CC, et al. Curr Biol. 2011 Oct 25;21(20):1704-15. doi: 10.1016/j.cub.2011.08.058. Epub 2011 Oct 13. Curr Biol. 2011. PMID: 22000105 Free PMC article. - Similarities of Drosophila rab GTPases based on expression profiling: completion and analysis of the rab-Gal4 kit.
Jin EJ, Chan CC, Agi E, Cherry S, Hanacik E, Buszczak M, Hiesinger PR. Jin EJ, et al. PLoS One. 2012;7(7):e40912. doi: 10.1371/journal.pone.0040912. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844416 Free PMC article. - Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes.
Fu Y, Zhu JY, Zhang F, Richman A, Zhao Z, Han Z. Fu Y, et al. Cell Tissue Res. 2017 Jun;368(3):615-627. doi: 10.1007/s00441-017-2575-2. Epub 2017 Feb 8. Cell Tissue Res. 2017. PMID: 28180992 Free PMC article. - Rapidly evolving Rab GTPase paralogs and reproductive isolation in Drosophila.
Hutter P. Hutter P. Adv Genet. 2007;58:1-23. doi: 10.1016/S0065-2660(06)58001-0. Adv Genet. 2007. PMID: 17452244 Review. - Rabs on the fly: Functions of Rab GTPases during development.
Caviglia S, Flores-Benitez D, Lattner J, Luschnig S, Brankatschk M. Caviglia S, et al. Small GTPases. 2019 Mar;10(2):89-98. doi: 10.1080/21541248.2017.1279725. Epub 2017 Feb 17. Small GTPases. 2019. PMID: 28118081 Free PMC article. Review.
Cited by
- Rab11 suppresses neuronal stress signaling by localizing dual leucine zipper kinase to axon terminals for protein turnover.
Kim SM, Quagraine Y, Singh M, Kim JH. Kim SM, et al. Elife. 2024 Oct 30;13:RP96592. doi: 10.7554/eLife.96592. Elife. 2024. PMID: 39475475 Free PMC article. - Ral inhibits ligand-independent Notch signaling in Drosophila.
Cho B, Fischer JA. Cho B, et al. Small GTPases. 2012 Jul-Sep;3(3):186-91. doi: 10.4161/sgtp.19802. Epub 2012 Jul 1. Small GTPases. 2012. PMID: 22750761 Free PMC article. - The synaptic vesicle SNARE neuronal Synaptobrevin promotes endolysosomal degradation and prevents neurodegeneration.
Haberman A, Williamson WR, Epstein D, Wang D, Rina S, Meinertzhagen IA, Hiesinger PR. Haberman A, et al. J Cell Biol. 2012 Jan 23;196(2):261-76. doi: 10.1083/jcb.201108088. J Cell Biol. 2012. PMID: 22270918 Free PMC article. - Ykt6-dependent endosomal recycling is required for Wnt secretion in the Drosophila wing epithelium.
Linnemannstöns K, Witte L, Karuna M P, Kittel JC, Danieli A, Müller D, Nitsch L, Honemann-Capito M, Grawe F, Wodarz A, Gross JC. Linnemannstöns K, et al. Development. 2020 Aug 14;147(15):dev185421. doi: 10.1242/dev.185421. Development. 2020. PMID: 32611603 Free PMC article. - α-Synuclein impairs macroautophagy: implications for Parkinson's disease.
Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O'Kane CJ, Rubinsztein DC. Winslow AR, et al. J Cell Biol. 2010 Sep 20;190(6):1023-37. doi: 10.1083/jcb.201003122. J Cell Biol. 2010. PMID: 20855506 Free PMC article.
References
- Ali, B. R., and M. C. Seabra, 2005. Targeting of Rab GTPases to cellular membranes. Biochem. Soc. Trans. 33(Pt 4): 652–656. - PubMed
- Amillet, J. M., D. Ferbus, F. X. Real, C. Antony, M. Mularis et al., 2006. Characterization of human Rab20 overexpressed in exocrine pancreatic carcinoma. Hum. Pathol. 37(3): 256–263. - PubMed
- Ayala, J., B. Olofsson, A. Tavitian and A. Prochiantz, 1989. Developmental and regional regulation of rab3: a new brain specific “ras-like” gene. J. Neurosci. Res. 22(3): 241–246. - PubMed
- Bao, S., J. Zhu and W. T. Garvey, 1998. Cloning of Rab GTPases expressed in human skeletal muscle: studies in insulin-resistant subjects. Horm. Metab. Res. 30(11): 656–662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials