Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden - PubMed (original) (raw)
Comparative Study
Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden
Tarja M Malm et al. J Neurosci. 2007.
Abstract
Pyrrolidine dithiocarbamate (PDTC) is a clinically tolerated inhibitor of nuclear factor-kappaB (NF-kappaB), antioxidant and antiinflammatory agent, which provides protection in brain ischemia models. In neonatal hypoxia-ischemia model, PDTC activates Akt and reduces activation of glycogen synthase kinase 3beta (GSK-3beta). Because chronic inflammation, oxidative stress, and increased GSK-3beta activity are features of Alzheimer's disease (AD) pathology, we tested whether PDTC reduces brain pathology and improves cognitive function in a transgenic animal model of AD. A 7 month oral treatment with PDTC prevented the decline in cognition in AD mice without altering beta-amyloid burden or gliosis. Moreover, marked oxidative stress and activation of NF-kappaB were not part of the brain pathology. Instead, the phosphorylated form of GSK-3beta was decreased in the AD mouse brain, and PDTC treatment increased the phosphorylation of Akt and GSK-3beta. Also, PDTC treatment increased the copper concentration in the brain. In addition, PDTC rescued cultured hippocampal neurons from the toxicity of oligomeric Abeta and reduced tau phosphorylation in the hippocampus of AD mice. Finally, astrocytic glutamate transporter GLT-1, known to be regulated by Akt pathway, was decreased in the transgenic AD mice but upregulated back to the wild-type levels by PDTC treatment. Thus, PDTC may improve spatial learning in AD by interfering with Akt-GSK pathway both in neurons and astrocytes. Because PDTC is capable of transferring external Cu2+ into a cell, and, in turn, Cu2+ is able to activate Akt, we hypothesize that PDTC provides the beneficial effect in transgenic AD mice through Cu2+-activated Akt pathway.
Figures
Figure 1.
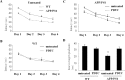
Long-term PDTC treatment prevented the cognitive decline of transgenic APP/PS1 mice. Test for acquisition was performed for 16-month-old transgenic APP/PSI mice and their wt controls after 7 months of PDTC treatment. Sixteen-month-old APP/PS1 mice were significantly slower in finding the platform when compared with wt controls as shown in A (ANOVA with repeated measures; F(1,68) = 7.0; p = 0.01). B, C, In wt mice, PDTC had no effect (B), whereas in transgenic mice, PDTC significantly improved the ability to locate the platform (C) (ANOVArm; F(1,31) = 6.6; p = 0.015). D, In the probe trial on day 4, PDTC did not affect the search bias of wt mice, whereas it significantly increased the time spent in the former platform quadrant of tg mice. Two tg mice were excluded as statistical outliers, one in the control and one in the PDTC group. **Significantly different from the wt control (p = 0.01; Dunnett's post hoc test). The final numbers of the animals per each group were as follows: wt, 17; wt PDTC, 19; tg, 15; and tg PDTC, 13. Error bars indicate SEM.
Figure 2.
DNA binding activity of NF-κB was not altered in APP/PS1 mice nor affected by PDTC treatment. A, B, Quantitative analysis (A) and a typical autoradiograph (B) of electrophoretic mobility shift assay of the DNA binding activity of NF-κB in wt and APP/PS1 transgenic mouse brain. The assay did not reveal any alterations in the DNA binding activity of NF-κB in APP/PS1 mice compared with their water-treated controls. PDTC treatment did not have any effect on the DNA binding activities (n = 8–10 per group). Error bars indicate SEM. C shows the specificity of the NF-κB complex formation (arrowhead) in control mice. The lanes are as follows: (1) normal assay, (2) 100× unlabeled competitor (cold consensus probe), (3) labeled mutated NF-κB binding probe, (4) consensus probe without protein, (5) supershift assay with specific anti-p50, (6) supershift assay with anti-p65, and (7) supershift assay with anti-YY1. The arrowheads show the specific NF-κB complex, the arrow shows the supershifted complex, and the asterisk shows the unspecific complex. The specific NF-κB complex contains both p50 and p65 proteins.
Figure 3.
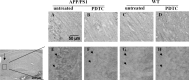
PDTC treatment had no effect on the brain Aβ burden. The effect of PDTC treatment on the brain Aβ burden was analyzed immunohistochemically by detecting diffuse Aβ deposition using pan-Aβ antibody staining and compact fibrillar plaques using thioflavin staining. The 16-month-old APP/PS1 mice exhibited extensive deposition of Aβ in the cortex detected by pan-Aβ antibody (A); however, quantification revealed that PDTC treatment had no effect either on the pan-Aβ immunoreactive areas (B, C) or thioflavin-stained area (D). Error bars indicate SEM.
Figure 4.
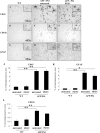
APP/PS1 mice exhibited markedly elevated gliosis which was not altered by PDTC treatment. Immunohistochemistry revealed significant gliosis as analyzed by microglial markers CD11b, CD45, and an astrocytic marker GFAP. A, D, and G show CD45, CD11b, and GFAP staining in frontal cortical area in wt mice, respectively. APP/PS1 mice had significant increased intensity of staining in all markers as shown in B, E, and H. PDTC treatment did not alter any of the glial markers analyzed (C, F, I). The high-power insets show typical morphology of stained glial cells. The quantification of each of the staining is shown in graphs J–L. Error bars indicate SEM. *p < 0.05; **p < 0.01.
Figure 5.
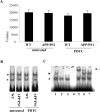
PDTC treatment increased phosphorylation of GSK-3β-Ser9. An illustration of typical immunoblots with antibodies against pGSK-3β-Ser9 and total GSK-3β and their subsequent quantification showed that the level of pGSK-3β-Ser9 was decreased in APP/PS1 mice compared with wt mice (A) and that PDTC treatment significantly increased the levels of pGSK-3β-Ser9 in APP/PS1 mouse brain (B). The differences in the level of pGSK-3β-Ser9 were not attributable to changes in total pGSK-3β, because the amount of total GSK-3β remained unchanged. Membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
Figure 6.
PDTC treatment increased phosphorylation of pAkt-Ser473. A, APP/PS1 mice had similar levels of pAkt-Ser473 compared with untreated wt mice. B, However, PDTC treatment significantly increased the levels of pAkt-Ser473. This increase was independent of the amount of total Akt, which remained unchanged in all animals. The membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
Figure 7.
PDTC treatment reduced the amount of phosphorylated tau in the hippocampus CA3 region of APP/PS1 mice. Photomicrographs showing AT8 immunoreactivity in the cortex (A–D) and CA3 pyramical cells (E–H) of wt and APP/PS1 mouse brains. A, AT8 immunoreactivity was observed surrounding putative Aβ deposits in the cortical areas of APP/PS1 mice, presumably reflecting dystrophic neurites. B–D, This staining was not clearly altered by PDTC treatment (B) and was absent in wt mice (C, D). E–H, High-power insets show AT8 immunoreactivity observed in the hippocampal CA3 region (arrow) pyramidal cells (arrowheads), in which APP/PS1 mice (E) exhibited more staining compared with wt mice (G). PDTC treatment did not alter the AT8 staining in wt mice (G, H); however, it decreased AT8 immunoreactivity in hippocampal CA3 region (F). The quantitative data are shown in Results. Scale bar, 50 μm.
Figure 8.
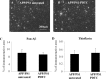
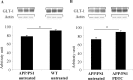
PDTC treatment increased the levels of astrocytic GLT-1. APP/PS1 mice exhibited a significant decrease in the level of astrocytic receptor GLT-1 (A). PDTC treatment significantly increased the levels of GLT-1, bringing it back to normal as depicted in B. The membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
Figure 9.
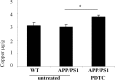
PDTC treatment increased the cortical copper concentration. Untreated APP/PS1 and wt mice exhibited a similar amount of copper in the cortex as measured by atomic absorption spectophotometer. Long-term PDTC treatment significantly increased the copper concentration in APP/PS1 mice. Error bars indicate SEM. *p < 0.05.
Figure 10.
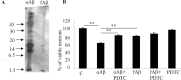
PDTC protected primary hippocampal neurons against oligomer-rich Aβ induced toxicity. A, Freshly dissolved Aβ preparation contained mostly oligomers, ranging from monomeric Aβ to high-molecular-weight forms. Fibrillization of Aβ resulted predominantly in high-molecular-weight aggregates that did not penetrate into the SDS-PAGE gel used in the current study. B, Exposure of primary hippocampal neurons to the freshly dissolved, oligomer-rich Aβ preparation caused ∼40% cell death as analyzed by the appearance of condensed chromatin. The cell death was significantly diminished by cotreatment with 1 μ
m
PDTC. Fibrillar Aβ was also toxic causing ∼20% cell death, although the effect was significantly smaller compared with oligomer-rich preparation. PDTC failed to protect the neurons against fibrillar Aβ toxicity. PDTC alone did not affect the cell viability. Error bars indicate SEM. *p < 0.01.
Similar articles
- Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia.
Nurmi A, Goldsteins G, Närväinen J, Pihlaja R, Ahtoniemi T, Gröhn O, Koistinaho J. Nurmi A, et al. Free Radic Biol Med. 2006 May 15;40(10):1776-84. doi: 10.1016/j.freeradbiomed.2006.01.011. Epub 2006 Feb 9. Free Radic Biol Med. 2006. PMID: 16678015 - Pyrrolidine dithiocarbamate activates the Nrf2 pathway in astrocytes.
Liddell JR, Lehtonen S, Duncan C, Keksa-Goldsteine V, Levonen AL, Goldsteins G, Malm T, White AR, Koistinaho J, Kanninen KM. Liddell JR, et al. J Neuroinflammation. 2016 Feb 26;13:49. doi: 10.1186/s12974-016-0515-9. J Neuroinflammation. 2016. PMID: 26920699 Free PMC article. - Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice.
Zhou Y, Xie N, Li L, Zou Y, Zhang X, Dong M. Zhou Y, et al. Int J Neuropsychopharmacol. 2014 Apr;17(4):635-44. doi: 10.1017/S146114571300148X. Epub 2013 Dec 18. Int J Neuropsychopharmacol. 2014. PMID: 24345484 - Tanshinone IIA regulates glycogen synthase kinase-3β-related signaling pathway and ameliorates memory impairment in APP/PS1 transgenic mice.
Peng X, Chen L, Wang Z, He Y, Ruganzu JB, Guo H, Zhang X, Ji S, Zheng L, Yang W. Peng X, et al. Eur J Pharmacol. 2022 Mar 5;918:174772. doi: 10.1016/j.ejphar.2022.174772. Epub 2022 Jan 25. Eur J Pharmacol. 2022. PMID: 35090935 - The role of metals in modulating metalloprotease activity in the AD brain.
Filiz G, Price KA, Caragounis A, Du T, Crouch PJ, White AR. Filiz G, et al. Eur Biophys J. 2008 Mar;37(3):315-21. doi: 10.1007/s00249-007-0244-1. Epub 2008 Feb 13. Eur Biophys J. 2008. PMID: 18270696 Review.
Cited by
- Altered biometal homeostasis is associated with CLN6 mRNA loss in mouse neuronal ceroid lipofuscinosis.
Kanninen KM, Grubman A, Caragounis A, Duncan C, Parker SJ, Lidgerwood GE, Volitakis I, Ganio G, Crouch PJ, White AR. Kanninen KM, et al. Biol Open. 2013 May 20;2(6):635-46. doi: 10.1242/bio.20134804. Print 2013 Jun 15. Biol Open. 2013. PMID: 23789114 Free PMC article. - Safety and neurochemical profiles of acute and sub-chronic oral treatment with agmatine sulfate.
Bergin DH, Jing Y, Williams G, Mockett BG, Zhang H, Abraham WC, Liu P. Bergin DH, et al. Sci Rep. 2019 Sep 3;9(1):12669. doi: 10.1038/s41598-019-49078-0. Sci Rep. 2019. PMID: 31481723 Free PMC article. - Phenazopyridine promotes RPS23RG1/Rps23rg1 transcription and ameliorates Alzheimer-associated phenotypes in mice.
Wang C, Zhang Y, Zhao D, Huo Y, Xie J, Zhang X, Luo H, Xu H, Zhang YW. Wang C, et al. Neuropsychopharmacology. 2022 Nov;47(12):2042-2050. doi: 10.1038/s41386-022-01373-7. Epub 2022 Jul 11. Neuropsychopharmacology. 2022. PMID: 35821069 Free PMC article. - Pharmacotherapeutic targets in Alzheimer's disease.
Biran Y, Masters CL, Barnham KJ, Bush AI, Adlard PA. Biran Y, et al. J Cell Mol Med. 2009 Jan;13(1):61-86. doi: 10.1111/j.1582-4934.2008.00595.x. Epub 2008 Nov 18. J Cell Mol Med. 2009. PMID: 19040415 Free PMC article. Review. - Cyp1B1 expression promotes angiogenesis by suppressing NF-κB activity.
Palenski TL, Gurel Z, Sorenson CM, Hankenson KD, Sheibani N. Palenski TL, et al. Am J Physiol Cell Physiol. 2013 Dec 1;305(11):C1170-84. doi: 10.1152/ajpcell.00139.2013. Epub 2013 Oct 2. Am J Physiol Cell Physiol. 2013. PMID: 24088896 Free PMC article.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Bayer TA, Schafer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schussel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA. 2003;100:14187–14192. - PMC - PubMed
- Beach TG, Kuo YM, Spiegel K, Emmerling MR, Sue LI, Kokjohn K, Roher AE. The cholinergic deficit coincides with Abeta deposition at the earliest histopathologic stages of Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:308–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases