Tracking Alzheimer's disease - PubMed (original) (raw)
Review
Tracking Alzheimer's disease
Paul M Thompson et al. Ann N Y Acad Sci. 2007 Feb.
Abstract
Population-based brain mapping provides great insight into the trajectory of aging and dementia, as well as brain changes that normally occur over the human life span. We describe three novel brain mapping techniques, cortical thickness mapping, tensor-based morphometry (TBM), and hippocampal surface modeling, which offer enormous power for measuring disease progression in drug trials, and shed light on the neuroscience of brain degeneration in Alzheimer's disease (AD) and mild cognitive impairment (MCI). We report the first time-lapse maps of cortical atrophy spreading dynamically in the living brain, based on averaging data from populations of subjects with Alzheimer's disease and normal subjects imaged longitudinally with MRI. These dynamic sequences show a rapidly advancing wave of cortical atrophy sweeping from limbic and temporal cortices into higher-order association and ultimately primary sensorimotor areas, in a pattern that correlates with cognitive decline. A complementary technique, TBM, reveals the 3D profile of atrophic rates, at each point in the brain. A third technique, hippocampal surface modeling, plots the profile of shape alterations across the hippocampal surface. The three techniques provide moderate to highly automated analyses of images, have been validated on hundreds of scans, and are sensitive to clinically relevant changes in individual patients and groups undergoing different drug treatments. We compare time-lapse maps of AD, MCI, and other dementias, correlate these changes with cognition, and relate them to similar time-lapse maps of childhood development, schizophrenia, and HIV-associated brain degeneration. Strengths and weaknesses of these different imaging measures for basic neuroscience and drug trials are discussed.
Figures
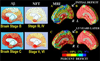
FIGURE 1
Gray matter deficits spread through the limbic system in moderate AD. Cortical atrophy occurring during the progression of AD is detected by comparing average profiles of gray matter between 12 AD patients (age: 68.4 ± 1.9 years) and 14 elderly matched controls (age: 71.4 ± 0.9 years). Average maps of gray matter density in patients and controls are subtracted at their first scan (when mean Mini-Mental State Exam (MMSE) score was 18 for the patients; [A] and [B]) and at their follow-up scan 1.5 years later (mean MMSE = 13; [C] and [D]). Colors show the average percentage loss of gray matter relative to the control average. Profound loss engulfs the left medial wall (>15%; [B] and [D]). On the right, however, the deficits in temporoparietal and entorhinal territory (A) spread forward into the cingulate gyrus 1.5 years later (C), after a 5-point drop in average MMSE. Limbic and frontal zones clearly show different degrees of impairment (C). The corpus callosum is indicated in white; maps of gray matter change are not defined here, as it is a white matter commissure. MRI-based changes, in living patients, agree strongly with the spatial progression of β-amyloid (Aβ) and NFT pathology observed post mortem (Braak Stages B,C and III to VI; left four panels adapted from Braak and Braak, 1997). The deficit sequence also matches the trajectory of NFT distribution observed post mortem, in patients with increasing dementia severity at death. Consistent with the deficit maps observed here, NFT accumulation is minimal in sensory and motor cortices, but occurs preferentially in entorhinal pyramidal cells, the limbic periallocortex (layers II/IV), the hippocampus/amygdala and subiculum, the basal forebrain cholinergic systems, and subsequently in temporoparietal and frontal association cortices (layers III/V)., Cortical layers III and V selectively lose large pyramidal neurons in association areas., This figure appears in color online.
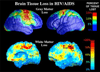
FIGURE 2
Visualizing brain tissue loss in HIV/AIDS. (Top row): In an MRI study of cortical thickness in 27 HIV/AIDS patients and 14 healthy controls, the primary sensory, motor, and premotor cortices were 15% thinner, and prefrontal and parietal tissue loss correlated with cognitive and motor deficits. Thinner frontopolar and language cortex also correlated with immune system deterioration measured via blood levels of CD4+ T-lymphocytes. (Bottom row): When the same subjects were studied using TBM, the pattern of white matter loss was in remarkable agreement with the cortical maps. The white matter volume was reduced in premotor areas where the cortex was significantly thinner, suggesting that cortical degeneration may be accompanied by degeneration in the underlying white matter pathways. Taken together, these and other studies support the notion that brain degeneration is present even in apparently healthy HIV-positive people on powerful drug regimens (HAART; highly active antiretroviral therapy). (Data in the top row are from Thompson et al., 2005; data in the bottom row are from Chiang et al., 2006).
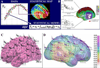
FIGURE 3
Mapping hippocampal atrophy. The 3D profile of hippocampal atrophy in disease can be mapped using surface-based modeling methods. The hippocampus is traced (A) either by hand or automatically, in serial coronal sections. After converting the traces to parametric surface mesh format (C) and (D), a medial core (i.e., a curve threading down the center of the hippocampus) is computed for each hippocampus. The distances from the medial core to each surface point are estimated and used to generate first individual and later average group distance maps. Here, group distance maps for elderly controls and patients with moderate Alzheimer’s disease are compared at baseline (left column), and after an approximately 2-year follow-up interval (right column). The significance maps show regions with significant atrophy at each time point (white colors). Similar maps can be made to plot regions where there is evidence for progressive atrophy over time or changes that link with cognitive test performance or clinical outcomes.
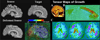
FIGURE 4
Mapping cortical changes. Several recent studies have examined the effects of age on the thickness of the cortex, measured in MRI scans of the brain.,,, This figure shows a general approach we developed to map cortical gray matter changes over time; it has also been used to produce time-lapse animations of the trajectory of cortical thinning with age, and to map the progression of diseases, such as childhood onset schizophrenia and Alzheimer’s disease.,, (A) First, measures (Yij) of gray matter thickness are obtained longitudinally (green dots) or once only (red dots) in a group of subjects at different ages. Fitting of statistical models to these data (Statistical Model, lower right) produces estimates of parameters that can be plotted onto the cortex, using a color code. These parameters can include age at peak (see arrow at peak of the curve), significance values, or estimated statistical parameters, such as rates of change, and effects of drug treatment or risk genes. (B) and (C): We estimated the trajectory of gray matter loss over the human life span in a cohort of 176 normal subjects. After cortical pattern matching was used to associate data from corresponding cortical regions, we developed software to fit a general nonlinear statistical model to the gray matter data from the population. This revealed significant nonlinear (quadratic) effects of time on brain structure. To show that it is feasible to pick up very small systematic differences using this technique, Sowell et al. (2006) found a sex difference in the trajectory of cortical thinning (D). There was an absolute excess in cortical thickness in women relative to men, of around 0.3 mm, mainly in the perisylvian language areas (even without brain size adjustments). Our other cortical thickness studies provide evidence for this sex difference, which is important to consider if cortical thickness is used to gauge degenerative brain changes. For full details of the approach, see Refs. ,. This figure appears in color online.
FIGURE 5
TBM maps rates of tissue gain or loss. Tensor-based morphometry, or TBM, can automatically map brain changes in large groups of subjects. Here, an example MRI image (top left) is aligned to a target image, and the shape of gyri, corpus callosum, and ventricles are well matched (data from Ref. 9). To better visualize the applied 3D deformation field, a colored grid is superimposed on the registered image—red and blue colors represent deformation orthogonal to the midsagittal plane (out of the page). (A) and (B): In Thompson et al. (2000), we used TBM to map growth rates (B) in the corpus callosum (A) of a young girl scanned at 3 years of age and again at 6 years—the anterior corpus callosum grows the fastest at this age (~20% local volume gain per year, most likely due to progressive myelination). Rates of tissue expansion are inferred from the derivatives of the warping field that aligns baseline to follow-up scans. (C): Progressive gray and white matter atrophy (purple colors) and ventricular expansion (red and white colors) are mapped in a patient with posterior cortical atrophy scanned 1, 1.5, and 2 years after diagnosis. After registering growth rate maps to a common neuroanatomical template, group differences in rates of brain change can be tested statistically (see Refs. and , for examples of studies comparing groups cross-sectionally and longitudinally). This figure appears in color online.
Similar articles
- Optimizing power to track brain degeneration in Alzheimer's disease and mild cognitive impairment with tensor-based morphometry: an ADNI study of 515 subjects.
Hua X, Lee S, Yanovsky I, Leow AD, Chou YY, Ho AJ, Gutman B, Toga AW, Jack CR Jr, Bernstein MA, Reiman EM, Harvey DJ, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM; Alzheimer's Disease Neuroimaging Initiative. Hua X, et al. Neuroimage. 2009 Dec;48(4):668-81. doi: 10.1016/j.neuroimage.2009.07.011. Epub 2009 Jul 14. Neuroimage. 2009. PMID: 19615450 Free PMC article. - Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls.
Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR Jr, Schuff N, Weiner MW, Thompson PM; Alzheimer's Disease Neuroimaging Initiative. Morra JH, et al. Neuroimage. 2009 Mar;45(1 Suppl):S3-15. doi: 10.1016/j.neuroimage.2008.10.043. Epub 2008 Nov 8. Neuroimage. 2009. PMID: 19041724 Free PMC article. - 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry.
Hua X, Leow AD, Lee S, Klunder AD, Toga AW, Lepore N, Chou YY, Brun C, Chiang MC, Barysheva M, Jack CR Jr, Bernstein MA, Britson PJ, Ward CP, Whitwell JL, Borowski B, Fleisher AS, Fox NC, Boyes RG, Barnes J, Harvey D, Kornak J, Schuff N, Boreta L, Alexander GE, Weiner MW, Thompson PM; Alzheimer's Disease Neuroimaging Initiative. Hua X, et al. Neuroimage. 2008 May 15;41(1):19-34. doi: 10.1016/j.neuroimage.2008.02.010. Epub 2008 Feb 21. Neuroimage. 2008. PMID: 18378167 Free PMC article. - The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and Alzheimer's disease/mild cognitive impairment: a review.
Kakeda S, Korogi Y. Kakeda S, et al. Neuroradiology. 2010 Aug;52(8):711-21. doi: 10.1007/s00234-010-0717-2. Epub 2010 May 22. Neuroradiology. 2010. PMID: 20495793 Review. - Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease.
Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. Teipel SJ, et al. Med Clin North Am. 2013 May;97(3):399-424. doi: 10.1016/j.mcna.2012.12.013. Epub 2013 Feb 1. Med Clin North Am. 2013. PMID: 23642578 Review.
Cited by
- Structural MRI in frontotemporal dementia: comparisons between hippocampal volumetry, tensor-based morphometry and voxel-based morphometry.
Muñoz-Ruiz MÁ, Hartikainen P, Koikkalainen J, Wolz R, Julkunen V, Niskanen E, Herukka SK, Kivipelto M, Vanninen R, Rueckert D, Liu Y, Lötjönen J, Soininen H. Muñoz-Ruiz MÁ, et al. PLoS One. 2012;7(12):e52531. doi: 10.1371/journal.pone.0052531. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23285078 Free PMC article. - Applying surface-based morphometry to study ventricular abnormalities of cognitively unimpaired subjects prior to clinically significant memory decline.
Dong Q, Zhang W, Stonnington CM, Wu J, Gutman BA, Chen K, Su Y, Baxter LC, Thompson PM, Reiman EM, Caselli RJ, Wang Y. Dong Q, et al. Neuroimage Clin. 2020;27:102338. doi: 10.1016/j.nicl.2020.102338. Epub 2020 Jul 5. Neuroimage Clin. 2020. PMID: 32683323 Free PMC article. - Personalized Diagnosis for Alzheimer's Disease.
Zhu Y, Kim M, Zhu X, Yan J, Kaufer D, Wu G. Zhu Y, et al. Med Image Comput Comput Assist Interv. 2017 Sep;10435:205-213. doi: 10.1007/978-3-319-66179-7_24. Epub 2017 Sep 4. Med Image Comput Comput Assist Interv. 2017. PMID: 30009282 Free PMC article. - Remote sites of structural atrophy predict later amyloid formation in a mouse model of Alzheimer's disease.
Badea A, Johnson GA, Jankowsky JL. Badea A, et al. Neuroimage. 2010 Apr 1;50(2):416-27. doi: 10.1016/j.neuroimage.2009.12.070. Epub 2009 Dec 24. Neuroimage. 2010. PMID: 20035883 Free PMC article. - Characterization of Atrophic Changes in the Cerebral Cortex Using Fractal Dimensional Analysis.
King RD, George AT, Jeon T, Hynan LS, Youn TS, Kennedy DN, Dickerson B; the Alzheimer’s Disease Neuroimaging Initiative. King RD, et al. Brain Imaging Behav. 2009 Jun;3(2):154-166. doi: 10.1007/s11682-008-9057-9. Brain Imaging Behav. 2009. PMID: 20740072 Free PMC article.
References
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr. Scand. 1987;76:465–479. Review. - PubMed
- Fox NC, Black RS, Gilman S, et al. AN1792(QS-21)-201 Study 2005. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 64:1563–1572. - PubMed
- Silverman DHS, Thompson PM. Structural and functional neuroimaging: focusing on mild cognitive impairment. Appl. Neurol. 2006;2:10–24.
- Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human lifespan. Nat. Neurosci. 2003;6:309–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DA15878/DA/NIDA NIH HHS/United States
- RR13642/RR/NCRR NIH HHS/United States
- R01DA017831/DA/NIDA NIH HHS/United States
- AG016570/AG/NIA NIH HHS/United States
- R21 RR019771/RR/NCRR NIH HHS/United States
- R01 LM005639/LM/NLM NIH HHS/United States
- HD050735/HD/NICHD NIH HHS/United States
- EB01651/EB/NIBIB NIH HHS/United States
- RR019771/RR/NCRR NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- R01 HD050735/HD/NICHD NIH HHS/United States
- P41 RR013642/RR/NCRR NIH HHS/United States
- U54 RR021813/RR/NCRR NIH HHS/United States
- RR021813/RR/NCRR NIH HHS/United States
- R21 DA015878/DA/NIDA NIH HHS/United States
- LM05639/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical