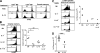Antiviral CD4+ memory T cells are IL-15 dependent - PubMed (original) (raw)
Antiviral CD4+ memory T cells are IL-15 dependent
Jared F Purton et al. J Exp Med. 2007.
Abstract
Survival and intermittent proliferation of memory CD4(+) and CD8(+) T cells appear to be controlled by different homeostatic mechanisms. In particular, contact with interleukin (IL)-15 has a decisive influence on memory CD8(+) cells, but not memory CD4(+) cells. Past studies of memory CD4(+) cells have relied heavily on the use of naturally occurring memory phenotype (MP) cells as a surrogate for antigen (Ag)-specific memory cells. However, we show here that MP CD4(+) cells contain a prominent subset of rapidly proliferating major histocompatibility complex (MHC) II-dependent cells. In contrast, Ag-specific memory CD4 cells have a slow turnover rate and are MHC II independent. In irradiated hosts, these latter cells ignore IL-15 and expand in response to the elevated levels of IL-7 in the lymphopenic hosts. In contrast, in normal nonlymphopenic hosts where IL-7 levels are low, memory CD4 cells are heavily dependent on IL-15. Significantly, memory CD4(+) responsiveness to endogenous IL-15 reflects marked competition from other cells, especially CD8(+) and natural killer cells, and increases considerably after removal of these cells. Therefore, under normal physiological conditions, homeostasis of CD8(+) and CD4(+) memory cells is quite similar and involves IL-15 and IL-7.
Figures
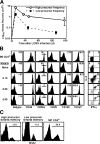
Figure 1.
Generation and characterization of Smarta memory cells. (A) The responses of naive Smarta CD4+ cells transferred into B6 hosts at a high or low precursor frequency. B6 mice were injected with 105 or 103 naive Thy-1.1+ Smarta cells and infected with LCMV Armstrong 1 d later. The recovery of Smarta cells in host spleen was analyzed at the indicated time points by staining for Thy-1.1 and CD4. (B) Characterization of Smarta naive, effector, and memory cells generated from 105 injected precursors. Histograms indicate expression of activation markers and cytokine receptors on Smarta cells before and after LCMV infection, shown in comparison with total polyclonal B6 CD4+ cells. Numbers inside histograms indicate mean fluorescence intensity. Dot plots show the expression of intracellular IL-2 and IFN-γ by Smarta cells after a 5-h in vitro stimulation with agonist GP61-80 peptide. Data are representative of four separate experiments, with at least three mice per time point. (C) Basal homeostatic proliferation rate of Smarta memory and CD4+ MP cells. B6 mice harboring a high or low precursor frequency of Smarta memory cells (at 72 d after LCMV infection) were given BrdU in the drinking water for 5 d, and the incorporation of BrdU on memory CD4+ cells was detected as described in Materials and methods. Similar results were obtained in two other experiments.
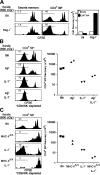
Figure 2.
MP CD4+ cells are comprised of heterogeneous populations of cells in terms of their homeostatic characteristics. (A) Comparison of acute homeostatic proliferation between Smarta memory and MP CD4+ cells. A mixture of CFSE-labeled Thy-1.1+ Smarta memory cells (4 × 105) and CD45.1+ MP CD4+ cells (2 × 105) was injected into irradiated B6 and Rag-1− mice, and the donor cells in the host spleen and LNs were analyzed 1 wk later by staining for Thy-1.1, CD45.1, and CD4. Histograms show CFSE profiles of the two donor cell types in the host spleen, and the bar graphs show mean donor cell recovery from pooled host spleen and LNs. Data are representative of two separate experiments, with two mice per host type analyzed individually. (B) MP CD4+ cells recognize a hybrid MHC II molecule in Aβ− mice. Small numbers (3 × 105) of CFSE-labeled purified Thy-1.1+ CD4+ MP cells were injected into irradiated and CD8/NK-depleted B6, Aβ−, IL-7−, and Aβ−IL-7− mice and were analyzed 1 wk later by staining splenocytes for CD4 and Thy-1.1. Aβ− B6 mice lack the H2-A β chain. CFSE dilution and recovery from one of two experiments, with two mice per treatment are shown. (C) The role of MHC II and IL-7 in expansion of MP CD4+ cells in lymphopenic hosts. A small dose (3 × 105) of purified MP CD4+ cells was injected into irradiated and CD8/NK cell–depleted B6, MHC-IIΔ/Δ, IL-7−, and MHC-IIΔ/Δ IL-7− mice. Donor cells from the host spleen were analyzed 1 wk later by staining for Thy-1.1 and CD4. MHC-IIΔ/Δ B6 mice lack all H2-A and E chains. Histograms and a log scale scatter-plot show proliferation and recovery of donor cells in the spleen. Data are representative of two separate experiments, with two mice per host type analyzed individually.
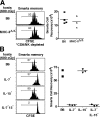
Figure 3.
IL-7, but not MHC II, is essential for acute homeostatic proliferation of Smarta memory cells. (A) Contact with MHC II is not required for acute homeostatic proliferation of Smarta memory cells. A small dose (3 × 105) of CFSE-labeled Thy-1.1+ Smarta memory cells was injected into irradiated, thymectomized, and CD8/NK cell–depleted B6 and MHC-IIΔ/Δ mice, and the donor cells in the host spleen and LNs were analyzed 1 wk later by staining for Thy-1.1 and CD4. The CFSE profiles and recoveries of donor Smarta cells are shown. Data are representative of two separate experiments, with three to four mice per host type analyzed individually. (B) Acute homeostatic proliferation of Smarta memory cells is chiefly driven by IL-7. A small dose (5 × 105) of CFSE-labeled Thy-1.1+ Smarta memory cells was injected into irradiated B6, IL-7−, IL-15−, and IL-7−15− mice, and host spleens were analyzed 2 wk later by staining for Thy-1.1 and CD4. The CFSE profiles and recoveries of donor Smarta cells are shown. Data are representative of three experiments, with two to three mice per host type analyzed individually.
Figure 4.
Ag-specific memory CD4+ cells require IL-15 to undergo homeostatic proliferation and to survive under normal nonirradiated conditions. (A) Exogenous IL-15 can induce slow proliferation of Smarta memory cells. A dose of 5 × 106 splenocytes from LCMV-primed B6 mice containing Thy-1.1+ Smarta memory cells was CFSE labeled and transferred to nonirradiated CD45.1+ B6 mice. Recipients were then injected with 1.5 μg of recombinant murine IL-15 or PBS on days 0 and 2, and the donor cells in the host spleen were analyzed on day 5 by staining for Thy-1.1, CD45.2, CD44, CD4, and CD8. The CFSE profiles of donor MP CD8+, MP CD4+, and Smarta memory cells are shown. Data are representative of two experiments using two recipients per treatment analyzed individually. (B) IL-15 is essential for basal homeostatic proliferation and survival of Smarta memory cells. A small dose (8 × 105) of CFSE-labeled Thy-1.1+ Smarta memory cells was injected into nonirradiated B6, IL-7−, IL-15−, and IL-7−IL-15− mice. Approximately 20% of the donor cells could be recovered from the spleen on day 1 in all hosts. Spleens were analyzed 51 d later by staining for Thy-1.1 and CD4, and the CFSE profiles and recovery of donor cells are shown. Data are representative of three experiments using two to four recipients per host type analyzed individually. *, P < 0.01; **, P < 0.05 by one-way ANOVA, Bonferroni's multiple comparison test. (C) Polyclonal-derived Ag-specific CD4+ secondary memory cells require IL-15 for their basal turnover. A dose of 2 × 107 purified CFSE-labeled CD4+ cells from Thy-1.1+ B6 mice previously immunized and boosted with LCMV (comprising ∼1.5 × 105 LCMV-specific polyclonal secondary memory CD4+ cells as detected by IFN-γ production) were injected into nonirradiated B6, IL-7−, IL-15−, and IL-7−IL-15− mice. The LCMV-specific polyclonal donor CD4+ memory cells were detected 51 d later by staining for Thy-1.1, CD4, and IFN-γ after a 5-h in vitro stimulation with agonist GP61-80 peptide. Data are representative of three experiments using two to four recipients per host type analyzed individually. (D) IL-15 sustains polyclonal-derived Ag-specific CD4+ memory cells. Experiments were performed as in C, except twofold higher numbers (3 × 105) of primary LCMV-specific polyclonal memory CD4+ cells were transferred into only B6 and IL-15− mice, and the recovery of donor cells was analyzed 65 d later. ***, P = 0.0127 by two-tailed unpaired t test.
Figure 5.
Smarta memory cells compete with CD8+ MP and NK cells for IL-15. (A) Comparative expression levels of CD122 and CD127 on various cell types. Representative histograms of CD122 and CD127 analyzed on CD4+ MP, Smarta memory, CD8+ MP, and NK cells from 3–4-mo-old B6 mice after staining for CD4, CD8, NK1.1, CD44, CD122, or CD127. Numbers indicate mean fluorescence intensity. (B) Depletion of CD8 and/or NK cells increases basal homeostatic proliferation rate of Smarta memory cells. A small dose (5 × 105) of CFSE-labeled Thy-1.1+ Smarta memory cells was injected into B6, IL-7−, or IL-15− mice that were treated with PBS or mAbs to deplete NK and/or CD8 cells. The CFSE profiles of donor cells analyzed 21 d later by staining for Thy-1.1 and CD4 are shown. As a comparison, CD8+ P14 memory cells undergoing basal homeostatic proliferation for 21 d in indicated hosts are shown. Data are representative of two experiments using two recipients per host type analyzed individually.
Similar articles
- Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells.
Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Tan JT, et al. J Exp Med. 2002 Jun 17;195(12):1523-32. doi: 10.1084/jem.20020066. J Exp Med. 2002. PMID: 12070280 Free PMC article. - Homeostasis of memory T cells.
Surh CD, Boyman O, Purton JF, Sprent J. Surh CD, et al. Immunol Rev. 2006 Jun;211:154-63. doi: 10.1111/j.0105-2896.2006.00401.x. Immunol Rev. 2006. PMID: 16824125 Review. - Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha.
Wherry EJ, Becker TC, Boone D, Kaja MK, Ma A, Ahmed R. Wherry EJ, et al. Adv Exp Med Biol. 2002;512:165-75. doi: 10.1007/978-1-4615-0757-4_22. Adv Exp Med Biol. 2002. PMID: 12405201 - Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help.
Choo DK, Murali-Krishna K, Anita R, Ahmed R. Choo DK, et al. J Immunol. 2010 Sep 15;185(6):3436-44. doi: 10.4049/jimmunol.1001421. Epub 2010 Aug 23. J Immunol. 2010. PMID: 20733203 - Generation and maintenance of memory CD4(+) T Cells.
van Leeuwen EM, Sprent J, Surh CD. van Leeuwen EM, et al. Curr Opin Immunol. 2009 Apr;21(2):167-72. doi: 10.1016/j.coi.2009.02.005. Epub 2009 Mar 11. Curr Opin Immunol. 2009. PMID: 19282163 Free PMC article. Review.
Cited by
- Co-administration of avian influenza virus H5 plasmid DNA with chicken IL-15 and IL-18 enhanced chickens immune responses.
Lim KL, Jazayeri SD, Yeap SK, Alitheen NB, Bejo MH, Ideris A, Omar AR. Lim KL, et al. BMC Vet Res. 2012 Aug 6;8:132. doi: 10.1186/1746-6148-8-132. BMC Vet Res. 2012. PMID: 22866758 Free PMC article. - Mapping autophagosome contents identifies interleukin-7 receptor-α as a key cargo modulating CD4+ T cell proliferation.
Zhou D, Borsa M, Puleston DJ, Zellner S, Capera J, Sanderson S, Schifferer M, Hester SS, Ge X, Fischer R, Jostins L, Behrends C, Alsaleh G, Simon AK. Zhou D, et al. Nat Commun. 2022 Sep 2;13(1):5174. doi: 10.1038/s41467-022-32718-x. Nat Commun. 2022. PMID: 36055998 Free PMC article. - Maintenance of the human memory T cell repertoire by subset and tissue site.
Miron M, Meng W, Rosenfeld AM, Dvorkin S, Poon MML, Lam N, Kumar BV, Louzoun Y, Luning Prak ET, Farber DL. Miron M, et al. Genome Med. 2021 Jun 14;13(1):100. doi: 10.1186/s13073-021-00918-7. Genome Med. 2021. PMID: 34127056 Free PMC article. - Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood.
Häringer B, Lozza L, Steckel B, Geginat J. Häringer B, et al. J Exp Med. 2009 May 11;206(5):1009-17. doi: 10.1084/jem.20082238. Epub 2009 May 4. J Exp Med. 2009. PMID: 19414553 Free PMC article. - Homeostasis of IL-15 dependent lymphocyte subsets in the liver.
Cepero-Donates Y, Rakotoarivelo V, Mayhue M, Ma A, Chen YG, Ramanathan S. Cepero-Donates Y, et al. Cytokine. 2016 Jun;82:95-101. doi: 10.1016/j.cyto.2015.12.012. Epub 2016 Jan 5. Cytokine. 2016. PMID: 26778709 Free PMC article.
References
- Sprent, J., and C.D. Surh. 2002. T cell memory. Annu. Rev. Immunol. 20:551–579. - PubMed
- Schluns, K.S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269–279. - PubMed
- Jameson, S.C. 2005. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin. Immunol. 17:231–237. - PubMed
- Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI064586/AI/NIAID NIH HHS/United States
- P01 AG001743/AG/NIA NIH HHS/United States
- R01 AI046710/AI/NIAID NIH HHS/United States
- AI046710/AI/NIAID NIH HHS/United States
- R01 AI045809/AI/NIAID NIH HHS/United States
- AG020186/AG/NIA NIH HHS/United States
- T32 AI007244/AI/NIAID NIH HHS/United States
- R01 AI064586/AI/NIAID NIH HHS/United States
- AI045809/AI/NIAID NIH HHS/United States
- R01 AG020186/AG/NIA NIH HHS/United States
- AI007244/AI/NIAID NIH HHS/United States
- AG001743/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous