Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection - PubMed (original) (raw)
Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection
Haina Shin et al. J Exp Med. 2007.
Abstract
Efficient maintenance of memory CD8 T cells is central to long-term protective immunity. IL-7- and IL-15-driven homeostatic proliferation is essential for long-term memory CD8 T cell persistence after acute infections. During chronic infections, however, virus-specific CD8 T cells respond poorly to these cytokines. Yet, virus-specific CD8 T cells often persist for long periods of time during chronic infections. We have addressed this apparent paradox by examining the mechanism for maintaining virus-specific CD8 T cells during chronic infection. We find that homeostatic cytokines (e.g., IL-7/15), inflammatory signals, and priming of recent thymic emigrants are not sufficient to maintain virus-specific CD8 T cells over time during chronic infection. Rather, our results demonstrate that viral peptide is required for virus-specific CD8 T cell persistence during chronic infection. Moreover, this viral antigen-dependent maintenance results in a dramatically different type of T cell division than is normally observed during memory T cell homeostasis. Rather than undergoing slow, steady homeostatic turnover during chronic viral infection, CD8 T cells undergo extensive peptide-dependent division, yet cell numbers remain relatively stable. These results indicate that antigen-specific CD8 T cell responses during persisting infection are maintained by a mechanism distinct from that after acute infection.
Figures
Figure 1.
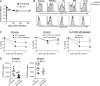
Virus-specific CD8 T cells do not require IL-7 and IL-15 to persist in chronically infected hosts. (A) Longitudinal analysis of Db/GP33-specific CD8 T cells in the PBMCs after LCMV Arm or clone 13 infection. n = 3–9/time point. (B) Analysis of CD127 and CD122 expression on Db/GP276+ CD8 T cells from tissues of Arm immune (> day 30 p.i.) and clone 13 (chronic)–infected mice (∼2–4 mo p.i.). Shaded histograms represents clone 13–infected mice, and lines represent Arm immune mice. All histograms are gated on Db/GP276+ CD8 T cells. Similar results were obtained for Db/GP33+ CD8 T cells (not depicted). (C) IL-15−/− and WT mice were infected with LCMV Arm or clone 13. After 2–3 mo, the IL-15−/− mice were treated with 200 μg αIL-7Rα antibody (Ab) i.p every 2–3 d for 2 wk, and the maintenance of virus-specific CD8 T cells was compared with untreated WT mice. Left plot represents Arm-infected αIL-7Rα–treated IL-15−/− and untreated WT mice. Middle plot represents clone 13–infected αIL-7Rα–treated IL-15−/− and untreated WT mice. Right plot displays a direct comparison of the same Arm and clone 13–infected αIL-7Rα–treated IL-15−/− groups from the first two graphs. All plots show Db/GP33-specific CD8 T cell frequency in the blood as a percentage of the Db/GP33-specific CD8 T cell frequency on the first day of treatment. Graphs are representative of three independent experiments; n = 8–11/time point. *, P < 0.05 by unpaired two-tailed t test. (D) Absolute number of Db/GP33-specific CD8 T cells in the spleens of Arm immune and clone 13–infected WT untreated and IL-15−/− αIL-7Rα–treated mice at the end of treatment. Graphs are representative of two independent experiments; n = 8–10/group. *, P = 0.03 by unpaired two-tailed t test. Error bars represent standard error of the mean.
Figure 2.
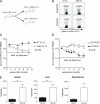
The V35A clone 13 variant virus causes chronic infection similar to WT clone 13. C57BL/6 mice were infected with WT or the V35A variant clone 13. (A) Virus levels were determined in the blood of mice infected with WT and the V35A variant clone 13 strains. (B) Tetramer staining of splenocytes on day 8 p.i. Plots are gated on CD8 T cells; numbers in quadrants indicate percentages of CD8 T cells staining with the indicated tetramer. (C) CD8 T cells from Arm immune (> day 30 p.i.) were adoptively transferred into V35A-infected Ly5.1+ B6 mice (∼2–7 mo p.i.). Donor Ly5.2+ Db/GP276+ and Db/GP33+ CD8 T cells were monitored in the blood. Kinetics of expansion was normalized to numbers at day 5 after transfer. n = 3–4 mice per time point. Data are representative of two independent experiments. (D) CFSE profiles are shown for the donor (Ly5.2+) Db/GP33 and Db/GP276-specific CD8 T cells in V35A recipients 3 wk after transfer for the experiment in part C. Plots are gated on CD8+ T cells. Histograms are gated on tetramer+ CD8 T cells. The numbers over the gates in the histograms represent percentages of tetramer+ CD8 T cells that have fully diluted their CFSE. Data are representative of two independent experiments. Error bars represent standard error of the mean.
Figure 3.
Virus-specific CD8 T cells from chronically infected mice do not persist without cognate antigen. (A) Schematic of experiments. Ly5.2+ C57BL/6 mice were infected with WT clone 13, and Ly5.1+ C57BL/6 were infected with either WT or V35A clone 13 on the same day. 2–3 mo later, after control of viremia, CD8 T cells were purified using magnetic beads from spleens of Ly5.2+ mice and equal numbers of Db/GP33+ CD8 T cells were transferred to Ly5.1+ WT or V35A clone 13–infected recipients. (B) Representative analysis of the Db/GP33+ and Db/GP276+ donor (Ly5.2+) CD8 T cells in the PBMCs of recipient mice. Numbers are the percentages of donor tetramer+ CD8 T cells. Plots are gated on Ly5.2+ CD8 T cells. (C) Frequency of Db/GP33+ donor cells in the blood of WT clone 13 and V35A clone 13–infected recipients over time. Difference between Db/GP33+ donor cells in WT clone 13 versus V35A clone 13 is significant by unpaired two-tailed t test at last time point (P = 0.008). Graph is representative of two independent experiments. (D) Frequency of Db/GP33+ and Db/GP276+ donor cells in the blood of V35A clone 13–infected recipients. Db/GP33+ CD8 T cells declined significantly in number over time (P < 0.05 by unpaired two-tailed t test). Difference between Db/GP33 and Db/GP276 frequency is also significant at all points after the initial bleed (P < 0.05 by unpaired two-tailed t test). Graph represents data from five independent experiments each with 2–3 mice/group. (E) Number of tetramer+ CD8 T cells in lymphoid and nonlymphoid tissues. BM represents two femurs. Graphs represent two to three independent experiments; n = 3–6/group. *, P < 0.04 by unpaired two-tailed t test. Difference between Db/GP33+ and Db/GP276+ populations in the bone marrow did not reach statistical significance (P = 0.09 by unpaired two-tailed t test). Dashed line represents limit of detection for parts C, D, and E. Error bars represent standard error of the mean.
Figure 4.
Virus-specific CD8 T cells are maintained by extensive proliferation during chronic infection. The experimental approach outlined in Fig. 3 A was used to monitor cell division history during chronic LCMV infection. (A) Proliferation patterns of donor virus-specific CD8 T cells in the blood. Left column indicates homeostatic proliferation of memory CD8 T cells from Arm immune mice (> day 30 p.i.) after adoptive transfer into naive mice. The middle column shows division of Db/GP33-specific CD8 T cells without antigen in the V35A-infected recipients, whereas the right column shows the division of the Db/GP276-specific CD8 T cell population with persisting antigen present in the same adoptive hosts. The donor CD8 T cells used for adoptive transferred were isolated from chronically infected donors 2–3 mo after infection. (B) Proliferation of WT clone 13–derived donor virus-specific CD8 T cells in the liver of V35A clone 13–infected recipients at 4 wk after transfer. (C) Histograms of donor tetramer+ CD8 T cells in the PBMCs at 4 wk after transfer. Numbers indicate the percentages of virus-specific CD8 T cells that have undergone no division (right gate), 1–5 divisions (middle gate), and 6+ divisions (left gate). All histograms are gated on Ly5.2+CD8+tetramer+ populations. (D) Graphs indicate the percentages of tetramer+ CD8 T cells that have undergone 0 divisions, 1–5 divisions, or 6+ divisions. Top two rows represents PBMCs, bottom row represents spleen, both at 4 wk after transfer. Panels are representative of two independent experiments for Arm immune and four independent experiments for chronic infection, each with n = 2–7 mice/group. A significantly greater percentage of Db/GP276+ CD8 T cells have undergone 6+ divisions in the spleen compared with Db/GP33+ CD8 T cells (P = 0.03 by unpaired two-tailed t test). The differences in the blood are also statistically significant with more Db/GP276+ CD8 T cells that have undergone 6+ divisions and fewer undivided compared with Db/GP33+ CD8 T cells (P = 0.03 and P = 0.02, respectively, by unpaired two-tailed t test). Error bars represent standard error of the mean.
Similar articles
- The role of CD80/CD86 in generation and maintenance of functional virus-specific CD8+ T cells in mice infected with lymphocytic choriomeningitis virus.
Grujic M, Bartholdy C, Remy M, Pinschewer DD, Christensen JP, Thomsen AR. Grujic M, et al. J Immunol. 2010 Aug 1;185(3):1730-43. doi: 10.4049/jimmunol.0903894. Epub 2010 Jul 2. J Immunol. 2010. PMID: 20601595 - IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections.
Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. Bahl K, et al. J Immunol. 2006 Apr 1;176(7):4284-95. doi: 10.4049/jimmunol.176.7.4284. J Immunol. 2006. PMID: 16547266 - CD8 T cell dysfunction during chronic viral infection.
Shin H, Wherry EJ. Shin H, et al. Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review. - Immune Memory and Exhaustion: Clinically Relevant Lessons from the LCMV Model.
Zehn D, Wherry EJ. Zehn D, et al. Adv Exp Med Biol. 2015;850:137-52. doi: 10.1007/978-3-319-15774-0_10. Adv Exp Med Biol. 2015. PMID: 26324351 Review.
Cited by
- Advances in T Cells Based on Inflammation in Metabolic Diseases.
Yu W, Li C, Zhang D, Li Z, Xia P, Liu X, Cai X, Yang P, Ling J, Zhang J, Zhang M, Yu P. Yu W, et al. Cells. 2022 Nov 10;11(22):3554. doi: 10.3390/cells11223554. Cells. 2022. PMID: 36428983 Free PMC article. Review. - T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion.
Utzschneider DT, Legat A, Fuertes Marraco SA, Carrié L, Luescher I, Speiser DE, Zehn D. Utzschneider DT, et al. Nat Immunol. 2013 Jun;14(6):603-10. doi: 10.1038/ni.2606. Epub 2013 May 5. Nat Immunol. 2013. PMID: 23644506 - Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination.
Kastenmuller W, Gasteiger G, Gronau JH, Baier R, Ljapoci R, Busch DH, Drexler I. Kastenmuller W, et al. J Exp Med. 2007 Sep 3;204(9):2187-98. doi: 10.1084/jem.20070489. Epub 2007 Aug 20. J Exp Med. 2007. PMID: 17709425 Free PMC article. - Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.
Studstill CJ, Hahm B. Studstill CJ, et al. Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review.
References
- Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. - PubMed
- Schluns, K.S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269–279. - PubMed
- Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials