Unrestrained erythroblast development in Nix-/- mice reveals a mechanism for apoptotic modulation of erythropoiesis - PubMed (original) (raw)
. 2007 Apr 17;104(16):6794-9.
doi: 10.1073/pnas.0610666104. Epub 2007 Apr 9.
Andrew G Koesters, Amy M Odley, Suvarnamala Pushkaran, Christopher P Baines, Benjamin T Spike, Diedre Daria, Anil G Jegga, Hartmut Geiger, Bruce J Aronow, Jeffery D Molkentin, Kay F Macleod, Theodosia A Kalfa, Gerald W Dorn 2nd
Affiliations
- PMID: 17420462
- PMCID: PMC1849960
- DOI: 10.1073/pnas.0610666104
Unrestrained erythroblast development in Nix-/- mice reveals a mechanism for apoptotic modulation of erythropoiesis
Abhinav Diwan et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Normal production of RBCs requires that the antiapoptotic protein Bcl-xl be induced at end stages of differentiation in response to erythropoietin (Epo) signaling. The critical proapoptotic pathways inhibited by Bcl-xl in erythroblasts are unknown. We used gene targeting in the mouse to evaluate the BH3-only factor Nix, which is transcriptionally up-regulated during Epo-stimulated in vitro erythrocyte differentiation. Nix null mice are viable and fertile. Peripheral blood counts revealed a profound reticulocytosis and thrombocytosis despite normal serum Epo levels and blood oxygen tension. Nix null mice exhibited massive splenomegaly, with splenic and bone marrow erythroblastosis and reduced apoptosis in vivo during erythrocyte maturation. Hematopoietic progenitor populations were unaffected. Cultured Nix null erythroid cells were hypersensitive to Epo and resistant to apoptosis stimulated by cytokine deprivation and calcium ionophore. Transcriptional profiling of Nix null spleens revealed increased expression of cell cycle and erythroid genes, including Bcl-xl, and diminished expression of cell death and B cell-related genes. Thus, cell-autonomous Nix-mediated apoptosis in opposition to the Epo-induced erythroblast survival pathway appears indispensable for regulation of erythrocyte production and maintenance of hematological homeostasis. These results suggest that physiological codependence and coordinated regulation of pro- and antiapoptotic Bcl2 family members may represent a general regulatory paradigm in hematopoiesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Nix effects on isolated mitochondria and general phenotype of Nix gene ablation in mice. (A) Isolated WT mouse liver mitochondria were incubated with increasing concentrations of GST-Nix, GST-sNix, and GST-Bax. Resultant mitochondrial pellet and supernatant underwent Western blotting for cytochrome c and cytochrome oxidase IV (COX-IV) (n = 3). (B) Time-course studies (0–180 min) as in A. (C) Swelling of isolated WT mitochondria induced by GST-Nix, 250 μM Ca2+, and GST-Nix + 250 μM Ca2+ (means of n = 2). (D) Schematic of Nix deletion strategy. Exons 1–6b (filled rectangles) and restriction sites are depicted (
see SI Methods
). (E) Southern blot (Left) and PCR (Right) screening of _Nix_-targeted mice. (F) Multiple-tissue Northern blot hybridized to Nix probes. (G) Hypomorphism. (H) Splenomegaly of WT (Upper) and _Nix_−/− (Lower) mice.
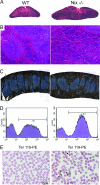
Fig. 2.
Splenic erythroblastosis and erythrocyte abnormalities in _Nix_−/− mice. (A and B) H&E-stained splenic sections. (Magnification: A, ×4; B, ×20.) (C) Ter119-stained (brown) splenic sections. Blue is counterstained lymphoid tissue. (D) Representative flow-cytometric quantification of Ter119+ splenocytes. (E) Wright-Giemsa-stained peripheral blood smears (1, polychromatic cells; 2, immature erythrocytes with redundant membrane; 3, discocytes).
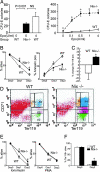
Fig. 3.
Erythroblast hyperplasia and diminished apoptosis in _Nix_−/− bone marrow and spleens. (A and B) Ter119 and CD71 expression in freshly isolated bone marrow (A) or splenic (B) cells. Cells: yellow, proerythroblasts (ProE); blue, basophilic erythroblasts (BasoE); pink, chromatophilic erythroblasts (ChromoE); green, orthochromatic erythroblasts (OrthoE). (C and D) Representative flow-cytometric analysis of Lin-, Sca-1/c-kit+ fraction (C), and Hoechst 33342-excluding “side population” (D) bone marrow cells. (E and F) Analysis of in vivo apoptosis in splenocytes. NonE, nonerythroblasts; ∗, P < 0.05.
Fig. 4.
Epo-hyperresponsiveness and apoptosis resistance of _Nix_−/− splenocytes. (A) CFU-E colony formation with and without increasing doses of Epo (n = 6–7 paired experiments; ∗, P ≤ 0.001 compared with WT). (B) Survival (Left, n = 5) and apoptosis (Right, n = 4) of splenocytes in monoculture. (C and D) Proportional change in Ter119+ splenocytes (C) and Ter119 and CD71 expression (D) after 48 h of suspension monoculture as in B (n = 4 paired experiments). (E) Splenocyte survival after apoptotic provocation with ionomycin 1 μg/ml (Left) or PMA 2 ng/ml (Right; n = 5 paired experiments; ∗, P < 0.05). (F) Survival of Ter119+ splenocytes in vitro (n = 4 paired experiments; ∗, P < 0.05).
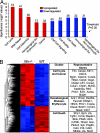
Fig. 5.
Altered patterns of gene expression in _Nix_−/− spleens. (A) Enrichment (red) and disenrichment (blue) of selected functional gene groups in _Nix_−/− spleens. (B) Dendrogram and heat map depiction (Left) and abbreviated list of regulated genes (Right). Color intensity (red:highest to blue:lowest) displays relative expression.
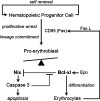
Fig. 6.
Schematic depiction of Nix involvement in erythroid maturation pathway. Fas L, Fas ligand.
Similar articles
- Targeting erythroblast-specific apoptosis in experimental anemia.
Diwan A, Koesters AG, Capella D, Geiger H, Kalfa TA, Dorn GW 2nd. Diwan A, et al. Apoptosis. 2008 Aug;13(8):1022-30. doi: 10.1007/s10495-008-0236-3. Apoptosis. 2008. PMID: 18584327 Free PMC article. - The BH3-only proteins BIM and PUMA are not critical for the reticulocyte apoptosis caused by loss of the pro-survival protein BCL-XL.
Delbridge AR, Aubrey BJ, Hyland C, Bernardini JP, Di Rago L, Garnier JM, Lessene G, Strasser A, Alexander WS, Grabow S. Delbridge AR, et al. Cell Death Dis. 2017 Jul 6;8(7):e2914. doi: 10.1038/cddis.2017.304. Cell Death Dis. 2017. PMID: 28682312 Free PMC article. - Homeostatic erythropoiesis by the transcription factor IRF2 through attenuation of type I interferon signaling.
Mizutani T, Tsuji K, Ebihara Y, Taki S, Ohba Y, Taniguchi T, Honda K. Mizutani T, et al. Exp Hematol. 2008 Mar;36(3):255-64. doi: 10.1016/j.exphem.2007.11.004. Epub 2008 Jan 22. Exp Hematol. 2008. PMID: 18207304 - Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria.
Chang KH, Stevenson MM. Chang KH, et al. Int J Parasitol. 2004 Dec;34(13-14):1501-16. doi: 10.1016/j.ijpara.2004.10.008. Int J Parasitol. 2004. PMID: 15582527 Review. - BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions.
Chinnadurai G, Vijayalingam S, Gibson SB. Chinnadurai G, et al. Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S114-27. doi: 10.1038/onc.2009.49. Oncogene. 2008. PMID: 19641497 Free PMC article. Review.
Cited by
- Mitophagy plays a "double-edged sword" role in the radiosensitivity of cancer cells.
Wang Q, Liu C. Wang Q, et al. J Cancer Res Clin Oncol. 2024 Jan 18;150(1):14. doi: 10.1007/s00432-023-05515-2. J Cancer Res Clin Oncol. 2024. PMID: 38238458 Free PMC article. Review. - Hypoxic adaptation of mitochondria and its impact on tumor cell function.
Benej M, Papandreou I, Denko NC. Benej M, et al. Semin Cancer Biol. 2024 May;100:28-38. doi: 10.1016/j.semcancer.2024.03.004. Epub 2024 Mar 30. Semin Cancer Biol. 2024. PMID: 38556040 Free PMC article. Review. - BNIP3L/NIX regulates both mitophagy and pexophagy.
Wilhelm LP, Zapata-Muñoz J, Villarejo-Zori B, Pellegrin S, Freire CM, Toye AM, Boya P, Ganley IG. Wilhelm LP, et al. EMBO J. 2022 Dec 15;41(24):e111115. doi: 10.15252/embj.2022111115. Epub 2022 Oct 10. EMBO J. 2022. PMID: 36215693 Free PMC article. - Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy.
Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW 2nd. Chen Y, et al. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9035-42. doi: 10.1073/pnas.0914013107. Epub 2010 Apr 23. Proc Natl Acad Sci U S A. 2010. PMID: 20418503 Free PMC article. - Role of BNIP3 and NIX in cell death, autophagy, and mitophagy.
Zhang J, Ney PA. Zhang J, et al. Cell Death Differ. 2009 Jul;16(7):939-46. doi: 10.1038/cdd.2009.16. Epub 2009 Feb 20. Cell Death Differ. 2009. PMID: 19229244 Free PMC article. Review.
References
- Wu H, Liu X, Jaenisch R, Lodish HF. Cell. 1995;83:59–67. - PubMed
- Koury MJ, Bondurant MC. Science. 1990;248:378–381. - PubMed
- Kelley LL, Koury MJ, Bondurant MC, Koury ST, Sawyer ST, Wickrema A. Blood. 1993;82:2340–2352. - PubMed
- Constantinescu SN, Ghaffari S, Lodish HF. Trends Endocrinol Metab. 1999;10:18–23. - PubMed
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Cell. 1999;98:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL069779/HL/NHLBI NIH HHS/United States
- HL77101/HL/NHLBI NIH HHS/United States
- HL69779/HL/NHLBI NIH HHS/United States
- R01 HL058010/HL/NHLBI NIH HHS/United States
- R01 HL059888/HL/NHLBI NIH HHS/United States
- R01 HL080262/HL/NHLBI NIH HHS/United States
- HL58010/HL/NHLBI NIH HHS/United States
- HL59888/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials