Significance of error-avoiding mechanisms for oxidative DNA damage in carcinogenesis - PubMed (original) (raw)
Review
Significance of error-avoiding mechanisms for oxidative DNA damage in carcinogenesis
Teruhisa Tsuzuki et al. Cancer Sci. 2007 Apr.
Abstract
Reactive oxygen species (ROS) are produced through normal cellular metabolism, and their formation is further enhanced by exposure to ionizing radiation and various chemicals. ROS attack DNA, and the resulting oxidative DNA damage is considered to contribute to aging, carcinogenesis and neurodegeneration. Among various types of oxidative DNA damage, 8-oxo-7,8-dihydroguanine (8-oxoguanine or 8-oxoG) is the most abundant, and plays significant roles in mutagenesis because of its ability to pair with adenine as well as cytosine. Enzymatic activities that may be responsible for preventing 8-oxoG-evoked mutations were identified in mammalian cells. We have focused on the following three enzymes: MTH1, OGG1 and MUTYH. MTH1 is a mammalian ortholog of Escherichia coli MutT, which hydrolyzes 8-oxo-dGTP to its monophosphate form in nucleotide pools, thereby preventing incorporation of the mutagenic substrate into DNA. OGG1, a functional counterpart of E. coli MutM, has an 8-oxoG DNA glycosylase activity. MUTYH, a mammalian ortholog of E. coli MutY, excises an adenine paired with 8-oxoG. These three enzymes are thought to prevent mutagenesis caused by 8-oxoG in mammals. To analyze the functions of mammalian MTH1 (Mth1), OGG1 (Ogg1) and MUTYH (Mutyh) in vivo, we established mutant mice for these three enzymes by targeted mutagenesis, and investigated spontaneous tumorigenesis as well as mutagenesis. Here we discuss our recent investigation of mutagenesis and carcinogenesis in these mutant mice.
Figures
Figure 1
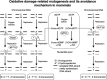
Mutations caused by oxidative damage. Genomes and their precursor nucleotides are constantly exposed to reactive oxygen species, which are generated by cellular metabolism or molecular executors in the host defense, and by environmental exposure to ionizing radiation and chemicals. To counteract this oxidative damage in nucleic acids, mammalian cells are equipped with three distinct enzymes. One is MTH1, a sanitizing enzyme for the nucleotide pool, and the others are DNA glycosylases, which remove mutagenic lesions in DNA. OGG1 excises 8‐oxo‐7,8‐dihydroguanine paired with cytosine in DNA, whereas MUTYH removes adenine paired with 8‐oxo‐7,8‐dihydroguanine and 1,2‐dihydro‐2‐oxoadenine paired with guanine in DNA.
Figure 2
Comparison of structures of MutT family proteins. Comparison of the predicted amino acid sequences of MutT homologs from humans,(12 ) mouse,(13 ) Escherichia coli,(19 ) Proteus vulgaris (55 ) and Streptococcus pneumoniae (56 ) are shown. Shaded boxes represent the regions with a high degree of amino acid sequence homology, with their amino acid sequences given below. The amino acid residues conserved through these five species are shown as white bold letters in black boxes. Numbers correspond to the positions of amino acid residues from the N‐termini. Asterisks indicate the residues that could not be replaced by any other residue without losing function.(20 ) Two mammalian MTH1 proteins are each composed of 156 amino acids. This highly conserved sequence motif, consisting of 23 amino acid residues, is now recognized as the Nudix box, shown on the bottom line as boldface letters.(21 ) Nudix hydrolases, a superfamily of Mg2+‐requiring enzymes, catalyze the hydrolysis of nucleoside diphosphates linked to other moieties, X, and contain the sequence motif or Nudix box, GX5EX7REUXEEXGU, where U is a bulky hydrophobic residue and X is any residue.
Figure 3
Oxidaive damage‐induced mutagenesis and avoidance mechanisms in mammals. Among the various types of oxidative damage in DNA, the oxidized forms of guanine and adenine, 8‐oxoguanine and 1,2‐dihydro‐2‐oxoadenine, can form relatively stable base pairs with adenine or guanine, respectively, in DNA. During DNA replication, they are thought to induce spontaneous mutagenesis, such as A : T to C : G and G : C to T : A transversions. The direct oxidation of DNA by reactive oxygen species has been reported to generate a substantial amount of 8‐oxo‐7,8‐dihydroguanine but little 1,2‐dihydro‐2‐oxoadenine. In contrast, 1,2‐dihydro‐2‐oxoadenine is generated exclusively by the oxidation of dATP in the nucleotide pool. Studies on mutator mutants have revealed that Escherichia coli has several error‐avoiding mechanisms that minimize the deleterious effects of 8‐oxo‐7,8‐dihydroguanine, and in which MutT, MutM and MutY proteins play important roles. MutT protein hydrolyzes 8‐oxo‐dGTP to 8‐oxo‐dGMT and pyrophosphate, thus avoiding the occurrence of A : T to C : G transversion mutations during DNA replication. MutM and MutY proteins are DNA glycosylases, the former excises 8‐oxoG paired with cytosine whereas the latter removes adenine paired with 8‐oxo‐7,8‐dihydroguanine. Mammalian cells are also equipped with elaborate error‐preventing mechanisms similar to those found in prokaryotes; MTH1 as a MutT homolog, OGG1 as a functional homolog for MutM, and MUTYH (MYH) as a MutY homolog. Recent studies showed that MTH1 effectively hydrolyzes 2‐OH‐dATP as well as 8‐oxo‐dGTP, and MUTYH has the ability to excise 1,2‐dihydro‐2‐oxoadenine inserted opposite guanine in the template strand as well as the ability to remove adenine incorporated opposite 8‐oxo‐7,8‐dihydroguanine in the template. As a result of the cooperative action of MTH1/OGG1/MUTYH and other repair pathways, mammalian cells effectively protect the occurrence of spontaneous mutations such as A : T to C : G and G : C to T : A transversions, which are caused by 8‐oxo‐7,8‐dihydroguanine and 1,2‐dihydro‐2‐oxoadenine.
Similar articles
- Functional cooperation of Ogg1 and Mutyh in preventing G: C-->T: a transversions in mice.
Isogawa A. Isogawa A. Fukuoka Igaku Zasshi. 2004 Jan;95(1):17-30. Fukuoka Igaku Zasshi. 2004. PMID: 15031996 - MTH1 as a nucleotide pool sanitizing enzyme: Friend or foe?
Nakabeppu Y, Ohta E, Abolhassani N. Nakabeppu Y, et al. Free Radic Biol Med. 2017 Jun;107:151-158. doi: 10.1016/j.freeradbiomed.2016.11.002. Epub 2016 Nov 7. Free Radic Biol Med. 2017. PMID: 27833032 Review. - The defense mechanisms in mammalian cells against oxidative damage in nucleic acids and their involvement in the suppression of mutagenesis and cell death.
Nakabeppu Y, Tsuchimoto D, Furuichi M, Sakumi K. Nakabeppu Y, et al. Free Radic Res. 2004 May;38(5):423-9. doi: 10.1080/10715760410001688348. Free Radic Res. 2004. PMID: 15293549 Review. - Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.
Boiteux S, Coste F, Castaing B. Boiteux S, et al. Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review. - Modulation of oxidative mutagenesis and carcinogenesis by polymorphic forms of human DNA repair enzymes.
Nohmi T, Kim SR, Yamada M. Nohmi T, et al. Mutat Res. 2005 Dec 11;591(1-2):60-73. doi: 10.1016/j.mrfmmm.2005.03.033. Epub 2005 Aug 2. Mutat Res. 2005. PMID: 16081110 Review.
Cited by
- Substrate specificity and excision kinetics of natural polymorphic variants and phosphomimetic mutants of human 8-oxoguanine-DNA glycosylase.
Sidorenko VS, Grollman AP, Jaruga P, Dizdaroglu M, Zharkov DO. Sidorenko VS, et al. FEBS J. 2009 Sep;276(18):5149-62. doi: 10.1111/j.1742-4658.2009.07212.x. Epub 2009 Aug 7. FEBS J. 2009. PMID: 19674107 Free PMC article. - DNA replication fidelity and cancer.
Preston BD, Albertson TM, Herr AJ. Preston BD, et al. Semin Cancer Biol. 2010 Oct;20(5):281-93. doi: 10.1016/j.semcancer.2010.10.009. Epub 2010 Oct 15. Semin Cancer Biol. 2010. PMID: 20951805 Free PMC article. Review. - Increased expression of versican in the inflammatory response to UVB- and reactive oxygen species-induced skin tumorigenesis.
Kunisada M, Yogianti F, Sakumi K, Ono R, Nakabeppu Y, Nishigori C. Kunisada M, et al. Am J Pathol. 2011 Dec;179(6):3056-65. doi: 10.1016/j.ajpath.2011.08.042. Epub 2011 Oct 12. Am J Pathol. 2011. PMID: 22001346 Free PMC article. - A model of oxidative stress management: moderation of carbohydrate metabolizing enzymes in SOD1-null Drosophila melanogaster.
Bernard KE, Parkes TL, Merritt TJ. Bernard KE, et al. PLoS One. 2011;6(9):e24518. doi: 10.1371/journal.pone.0024518. Epub 2011 Sep 1. PLoS One. 2011. PMID: 21909438 Free PMC article. - Role of Bacillus subtilis error prevention oxidized guanine system in counteracting hexavalent chromium-promoted oxidative DNA damage.
Santos-Escobar F, Gutiérrez-Corona JF, Pedraza-Reyes M. Santos-Escobar F, et al. Appl Environ Microbiol. 2014 Sep;80(17):5493-502. doi: 10.1128/AEM.01665-14. Epub 2014 Jun 27. Appl Environ Microbiol. 2014. PMID: 24973075 Free PMC article.
References
- Gajewski E, Rao G, Nackerdien Z et al. Modification of DNA bases in mammalian chromatin by radiation‐generated free radicals. Biochemistry 1990; 29: 7876–82. - PubMed
- Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res 1991; 250: 3–16. - PubMed
- Kasai H, Tanooka H, Nishimura S. Formation of 8‐hydroxyguanine residues in DNA by X‐irradiation. Gann 1984; 75: 1037–9. - PubMed
- Grollman AP, Moriya M. Mutagenesis by 8‐oxoguanine: an enemy within. Trends Genet 1993; 9: 246–9. - PubMed
- Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation‐damaged base 8‐oxoG. Nature 1991; 349: 431–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials