Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association - PubMed (original) (raw)
Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association
Pablo Monetta et al. Mol Biol Cell. 2007 Jul.
Abstract
Assembly of the cytosolic coat protein I (COPI) complex at the ER-Golgi interface is directed by the ADP ribosylation factor1 (Arf1) and its guanine nucleotide exchange factor (GBF1). Rab1b GTPase modulates COPI recruitment, but the molecular mechanism underlying this action remains unclear. Our data reveal that in vivo expression of the GTP-restricted Rab1b mutant (Rab1Q67L) increased the association of GBF1 and COPI to peripheral structures localized at the ER exit sites (ERES) interface. Active Rab1b also stabilized Arf1 on Golgi membranes. Furthermore, we characterized GBF1 as a new Rab1b effector, and showed that its N-terminal domain was involved in this interaction. Rab1b small interfering RNA oligonucleotide assays suggested that Rab1b was required for GBF1 membrane association. To further understand how Rab1b functions in ER-to-Golgi transport, we analyzed GFP-Rab1b dynamics in HeLa cells. Time-lapse microscopy indicated that the majority of the Rab1b-labeled punctuated structures are relatively short-lived with limited-range movements. FRAP of Golgi GFP-Rab1bwt showed rapid recovery (t(1/2) 120 s) with minimal dependence on microtubules. Our data support a model where Rab1b-GTP induces GBF1 recruitment at the ERES interface and at the Golgi complex where it is required for COPII/COPI exchange or COPI vesicle formation, respectively.
Figures
Figure 1.
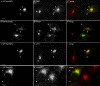
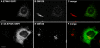
Rab1b-GTP enhances membrane association of GBF1 and COPI at the ERES interface. HeLa cells were transfected with GFP-Rab1Q67L, and analyzed 60 h after transfection. Panels show IF assays to detect GFP-Rab1Q67L and GBF1 (A–C), β-COP (D–F), p115 (G–I), and ERGIC53 (J–L). Asterisks label untransfected cells. Bars, 10 μm.
Figure 2.
Immunofluorescence of GFP-Rab1b–transfected cells. IF assays of HeLa cells transiently transfected over 24 h. Panels show IF assays to detect GFP-Rab1bwt and Sec23 (A–C); ERGIC53 (D–F), β-COP (G–I), and GBF1 (J–L). Arrowheads in the respective merged panel show colocalization in between GFP-Rab1b and each marker. Bars, 10 μm.
Figure 3.
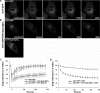
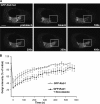
Kinetics of Arf1-GFP binding to and dissociation from Golgi membranes in cells expressing Rab1b-GTP. (A and B) Representative FRAP experiments in HeLa cells transiently expressing Arf1-GFP or coexpressing Arf1-GFP and CFP-Rab1Q67L. The Golgi was selectively photobleached (white square). The first frames show an initial prebleached image. After photobleaching, images frames were selected at times 0, 50, 100, and 200 s. Bars, 10 μm. Bottom panel in B shows expression of CFP-Rab1Q67L in the same cell. Note that the CFP signal is not recognized in the GFP detection conditions. (C) Quantification of Golgi intensity in Arf1-GFP–expressing cells (○; n = 5), Arf1-GFP/CFP-Rab1Q67L (▴; n = 4), and Arf1-GFP/CFP-Rab1bwt–coexpressing cells (•; n = 3). Error bars, SD. (D) Quantification of Golgi intensity in Arf1-GFP–expressing cells (▴; n = 7) and in Arf1-GFP/CFP-Rab1Q67L–coexpressing cells (○; n = 4) during 500 s after BFA (5 μg/ml) treatment. Error bars, SD.
Figure 4.
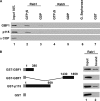
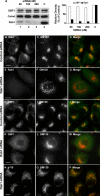
GBF1 preferentially binds to Rab1b-GTP. (A) GST pulldown assays performed with similar quantities of GST-Rab1b and GST-Rab5 loaded with GDP or GTPγS incubated in the presence of rat liver cytosol (lanes 2–5). Glutathione-Sepharose (lane 6) and GST alone (lane 7) were used as controls. Panels show Western blot analysis of bound fractions probed with anti-GBF1, anti-p115, and anti-αCOP. (B) Purified recombinant Rab1b loaded with GTPγS was incubated with truncations of GBF1 (1-380 or 1430-1859 aa) or full-length p115 fused to GST, as well as GST alone. Rab1b bound to GST proteins was detected by Western blot probed with anti-Rab1b (lane 2). Lanes 1 (A and B) represent 10% of input.
Figure 5.
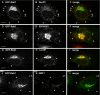
Role of the N-terminal domain in GBF1-E794K mutant intracellular localization. (A–C) Cell expressing GBF1 dominant negative construct E794K-Myc. (D–F) Cell expressing E794K-Myc without 294 aa from the N-terminal domain (ΔN-E794K GBF1). (B and E) The Golgi pattern, labeled with GM130, in transfected and untransfected cells. Bars, 10 μm.
Figure 6.
Rab1b requirement for GBF1 membrane association. RNAi treatment to knock down Rab1b levels in HeLa cells. (A) Western blot (and its quantification) performed after 72 h of transfection with different amounts of Rab1b siRNAs (lanes 1–3) or with control siRNAs (lane 4), probed with anti-Rab1b, anti-GBF1, or anti-Calreticulin as a loading control. The density of Rab1b and GBF1 bands relative to the density of Calreticulin was calculated. Relative density in controls was taken as 100%. (B–P) Subcellular localization of Rab1b, GBF1, GM130, and p115 in either Rab1b-silenced (100 nM Rab1b siRNA) or control cells (indicated at left). Bars, 10 μm.
Figure 7.
Dynamics of Rab1b labeled peripheral structures. (A and B) Time-lapse imaging of peripheral structures labeled with GFP-Rab1bwt transiently expressed in HeLa cells. (A) A representative cell is shown. Image frames were selected from the associated Quick Time movie every 60 s from time 0 to 420 s. Arrowheads indicate two immobile structures. White boxes indicate selected regions shown in B. Bars, 10 μm. (B) Different behaviors of mobile and transient peripheral structures. Regions 1 and 2 show mobile structures that either described peripheral long-range random movements or emerged from the cell periphery and moved toward the Golgi (arrowheads). Region 3 shows a transient structure that appeared and then vanished (arrowheads) during a short time period. Image frames were selected from the associated Quick Time movie at the indicated times. Bars, 5 μm.
Figure 8.
Kinetics of Rab1b binding to and dissociation from Golgi membranes. (A) Representative FRAP experiment in HeLa cell transiently expressing GFP-Rab1bwt. (B) Quantification of GFP-Rab1bwt FRAP from two independent experiments, with 5 μg/ml nocodazole (▴; n = 4 cells) or without nocodazole (○; n = 5 cells). Error bars, SD.
Similar articles
- COPI recruitment is modulated by a Rab1b-dependent mechanism.
Alvarez C, Garcia-Mata R, Brandon E, Sztul E. Alvarez C, et al. Mol Biol Cell. 2003 May;14(5):2116-27. doi: 10.1091/mbc.e02-09-0625. Epub 2003 Feb 6. Mol Biol Cell. 2003. PMID: 12802079 Free PMC article. - Modeling the dynamic behaviors of the COPI vesicle formation regulators, the small GTPase Arf1 and its activating Sec7 guanine nucleotide exchange factor GBF1 on Golgi membranes.
Sager G, Szul T, Lee E, Kawai R, Presley JF, Sztul E. Sager G, et al. Mol Biol Cell. 2021 Mar 1;32(5):446-459. doi: 10.1091/mbc.E20-09-0587. Epub 2021 Jan 6. Mol Biol Cell. 2021. PMID: 33405949 Free PMC article. - Spatial-Temporal Study of Rab1b Dynamics and Function at the ER-Golgi Interface.
Martinez H, García IA, Sampieri L, Alvarez C. Martinez H, et al. PLoS One. 2016 Aug 8;11(8):e0160838. doi: 10.1371/journal.pone.0160838. eCollection 2016. PLoS One. 2016. PMID: 27500526 Free PMC article. - Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.
Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Antonny B, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review. - GBF1 and Arf1 function in vesicular trafficking, lipid homoeostasis and organelle dynamics.
Kaczmarek B, Verbavatz JM, Jackson CL. Kaczmarek B, et al. Biol Cell. 2017 Dec;109(12):391-399. doi: 10.1111/boc.201700042. Epub 2017 Nov 6. Biol Cell. 2017. PMID: 28985001 Review.
Cited by
- Viral reorganization of the secretory pathway generates distinct organelles for RNA replication.
Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, Cameron CE, Ehrenfeld E, van Kuppeveld FJ, Altan-Bonnet N. Hsu NY, et al. Cell. 2010 May 28;141(5):799-811. doi: 10.1016/j.cell.2010.03.050. Cell. 2010. PMID: 20510927 Free PMC article. - Binding of the vesicle docking protein p115 to the GTPase Rab1b regulates membrane recruitment of the COPI vesicle coat.
Guo Y, Linstedt AD. Guo Y, et al. Cell Logist. 2014 Jan 9;3:e27687. doi: 10.4161/cl.27687. eCollection 2013. Cell Logist. 2014. PMID: 25332841 Free PMC article. - Rab1 recruits WHAMM during membrane remodeling but limits actin nucleation.
Russo AJ, Mathiowetz AJ, Hong S, Welch MD, Campellone KG. Russo AJ, et al. Mol Biol Cell. 2016 Mar 15;27(6):967-78. doi: 10.1091/mbc.E15-07-0508. Epub 2016 Jan 28. Mol Biol Cell. 2016. PMID: 26823012 Free PMC article. - Role of the Mosaic Cisternal Maturation Machinery in Glycan Synthesis and Oncogenesis.
Sahu P, Balakrishnan A, Di Martino R, Luini A, Russo D. Sahu P, et al. Front Cell Dev Biol. 2022 Apr 6;10:842448. doi: 10.3389/fcell.2022.842448. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35465326 Free PMC article. Review. - β-Arrestin2 Is Critically Involved in the Differential Regulation of Phosphosignaling Pathways by Thyrotropin-Releasing Hormone and Taltirelin.
Drastichova Z, Trubacova R, Novotny J. Drastichova Z, et al. Cells. 2022 Apr 27;11(9):1473. doi: 10.3390/cells11091473. Cells. 2022. PMID: 35563779 Free PMC article.
References
- Allan B. B., Moyer B. D., Balch W. E. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. - PubMed
- Alvarez C., Garcia-Mata R., Hauri H. P., Sztul E. The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 2001;276:2693–2700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous