Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK - PubMed (original) (raw)
Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK
Rebecca Stockton et al. Mol Biol Cell. 2007 Jun.
Abstract
Increased permeability of blood vessels is an important component of inflammation, but in some circumstances it contributes to tissue injury and organ failure. Previous work showed that p21-activated kinase (PAK) is a critical regulator of endothelial cell-cell junctions through effects on myosin light chain phosphorylation and cell contractility. We now show that blocking PAK function inhibits fluid leak in a mouse model of acute lung injury. In cultured endothelial cells, induction of myosin light chain phosphorylation by PAK is mediated by mitogen-activated protein kinase kinase and extracellular signal-regulated kinase (Erk). Erk in lipopolysaccharide (LPS)-treated mouse lung is activated in a PAK-dependent manner in several cell types, most prominently vascular endothelium. Activation of Erk requires the integrity of the complex between PAK, PIX, and GIT1. Several means of disrupting this complex inhibit stimulation of vascular permeability in vitro. A cell-permeant peptide that blocks binding of PAK to PIX inhibits LPS-induced fluid leak in the mouse lung injury model. We conclude that the PAK-PIX-GIT1 complex is critical for Erk-dependent myosin phosphorylation and vascular permeability.
Figures
Figure 1.
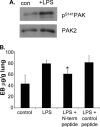
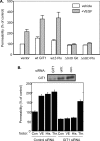
Role of PAK in vascular permeability in a mouse model of lung inflammation. (A) Mice inhaled aerosolized LPS for 30 min, with or without prior intraperitoneal injection of 1 mg of PAK N-terminal peptide or mutated, control peptide. Control animals inhaled saline. At 6 h, lungs were removed, extracted, and analyzed by Western blotting for pSer141 and total PAK2. Two experiments gave similar results. (B) Mice were injected with control or PAK N-terminal peptide and exposed to LPS or saline as described in A. At 6 h, microvascular permeability was determined leakage of Evans blue dye (EB) as described in Materials and Methods. Values are means ± SD, n = at least 4. *p < 0.01, relative to LPS-treated mice without peptides.
Figure 2.
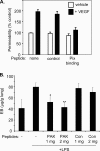
Erk mediates effects on permeability downstream of PAK. (A) BAECs were pretreated with 20 μg/ml PAK N-terminal (NT) peptide that blocks Nck binding; a mutated, control peptide; or the MEK inhibitor U0126 (25 μM). After 1 h, 25 ng/ml VEGF was added for 30 min. Cells were then fixed and stained for activated Erk. (B) Cells as described in A were extracted and analyzed for Erk phosphorylation by Western blotting. Quantitation: values are means ± SE for phospho-Erk normalized total Erk (n = 3). The increase after VEGF in the control sample is statistically significant, as are the differences between VEGF-induced permeability in control versus PAK peptide, and between control and UO126 (p < 0.01 in all cases). (C) BAECs on 3-μm filters were transfected with dominant-negative MEK1 (DN MEK), pretreated for 1 h with 20 μg/ml PAK N-terminal peptide or mutated, control peptide, or pretreated with 25 μM U0126. Cells were left untreated or stimulated for 1 h with 25 ng/ml VEGF, 50 ng/ml bFGF, or 10 μM histamine. Leakage of HRP across the filter was assayed as described in Materials and Methods. Values are means ± SD, n = 3.
Figure 3.
PAK-independent effects of LPS and EGF. (A) BAECs were treated with LPS or EGF at the indicated concentrations for 30 min and then lysed and analyzed by Western blotting for PAK phosphorylation, Erk phosphorylation, and total Erk as a loading control. Similar results were obtained in 3 independent experiments. (B) BAECs were pretreated the inhibitory peptide described in Figure 7 (PIX-blocking peptide; PBP) or inactive control peptide (Con) at 20 μg/ml. They were then stimulated with LPS or EGF. After 30 min, cells were lysed and analyzed by Western blotting for phosphorylated Erk or total Erk. Similar results were obtained in three independent experiments. (C) BAECs on filters were pretreated with PIX-blocking (PB) peptide or control peptide as in Figure 3B and then stimulated with 10 ng/ml LPS, 10 ng/ml EGF, or 25 ng/ml bFGF as indicated. Permeability across the filter was assayed by movement of HRP as described in Materials and Methods. Values are means ± SD, n = 3.
Figure 4.
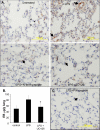
Erk activation in mice after LPS inhalation. (A) Mice were injected intraperitoneally with 1 mg of PAK N-terminal peptide, 1 mg of control peptide, or 0.5 ml of UO126 at 70 μM, and then they were treated with saline or LPS as described in Figure 1. At 6 h, the lungs were removed, fixed, and embedded. They then were sectioned and stained for activated Erk by using pT202/pY204 antibody. Small arrows indicate unidentified cells in control lungs that stain positively. Large arrows indicate conduit blood vessels. (B) Mice were treated with saline or LPS, with or without prior injection of 0.5 ml of MEK inhibitor U0126 at 70 μM. At 6 h, leakage of Evans blue dye (EB) was assayed as described in A. Asterisk (*) indicates statistical significance, p < 0.05 relative to LPS-treated mice without UO126; n ≥4. (C) Some mice from the same experiment, described in A were injected with 1 mg/ml PIX blocking peptide, exposed to LPS and sections stained for phospho-Erk. Arrow indicates a conduit vessel.
Figure 5.
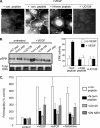
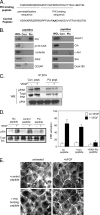
Requirements for βPIX and GIT1 in PAK-dependent permeability. (A) BAECs stimulated with 25 ng/ml VEGF for 30 min were fixed and immunostained for GIT1 or βPIX. (B) BAECs were left untreated or stimulated with VEGF and then extracted and immunoprecipitated with anti-phospho-S141 PAK antibody. The IPs were probes for phospho-MEK1/2, or, as a loading control, for phospho-PAK (pPAK). HC, IgG heavy chain. (C) BAECs were transfected by nucleoporation, using a protocol that gives 80–90% transfection efficiency, with WT PIX or ΔGBD PIX. Starved cells were untreated or stimulated with VEGF as described in A, extracted, and PIX immunoprecipitated. The presence of PIX and active MEK in the IPs was determined by Western blotting. (D) BAECs were nucleoporated with WT GIT1, GIT1 with a SPA2 homology domain (SHD) deletion that does not bind βPIX, WT βPIX, or ΔGBD βPIX. Cells were stimulated with VEGF as described in A and then fixed and stained for phospho-S141 PAK. (E and F) Cells were transfected and stimulated with VEGF as in A, then detergent extracted and analyzed by Western blotting to detect Erk phosphorylation (E) and MLC ser19 phosphorylation (F). (G) Quantitation. For Erk phosphorylation in E, values are means ± SE, n = 3, normalized for total proteins levels. Stimulation by VEGF was statistically significant (p < 0.01) as was the decrease in Erk activity after VEGF for GIT1 ΔSHD (p < 0.01) and PIX ΔGBD (p < 0.03) compared with vector control. For MLC, values are means ± range, n = 2, normalized to total protein levels. Stimulation by VEGF was statistically significant (p < 0.025) as was the decrease in Erk activity after VEGF for GIT1 ΔSHD and PIX ΔGBD compared with vector control (p < 0.04 for both).
Figure 6.
GIT1 and βPIX control permeability in vitro. (A) Cells transfected as in Figure 4C were plated on filters with 3-μm pores at confluent density. Cultures were stimulated with VEGF, and permeability to HRP was assayed as described in Materials and Methods. (B) HUVECs were transfected with siRNA oligonucleotides specific to GIT1 or with control, scrambled siRNA. Total cell lysates at 72 h were analyzed for GIT1 expression by Western blotting (top). Cells on filters were analyzed for permeability to HRP after treatment with 50 ng/ml VEGF, 10 μM histamine, or 0.2 U/ml thrombin.
Figure 7.
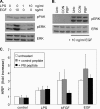
An inhibitory peptide blocks the interaction between PAK and PIX. (A) Sequence of the PIX SH3-blocking peptide and the mutated control. (B) Cell lysates were incubated with PIX-blocking (PIX) or control (Con) peptides immobilized on streptavidin beads. The beads were rinsed, and the bound proteins or whole cell lysates (WCL) or analyzed. Western blots were probed for the indicated SH3 domain-containing proteins. (C) BAECs were incubated with PIX-blocking or control peptide at 20 μg/ml for 1 h and then stimulated with VEGF for 30 min. Cells were rinsed, lysed, and βPIX immunoprecipitated. The IPs were analyzed by Western blotting for PIX as a control, or for PAK and phospho-PAK (pPAK) to detect coIP. (D) BAECs were incubated with peptides and stimulated with VEGF as described in C and then lysed and analyzed for Erk activation by Western blotting against phospho-Erk. Total Erk was analyzed to demonstrate equal loading. Values are means ± SE, n = 3, normalized to total protein. VEGF stimulation of Erk phosphorylation was statistically significant, as was the inhibition byGIT Δ SHD (p < 0.02). (E) Cells were incubated with PIX-blocking or control peptides as described in C, stimulated with bFGF for 60 min, and then stained for F-actin.
Figure 8.
Peptide blocking of the PIX–PAK interaction inhibits permeability. (A) BAECs on filters with 3-μm pores were pretreated with 20 μg/ml PIX-blocking or control peptides for 1 h and then stimulated with VEGF for 30 min. HRP movement across the monolayer was assayed as described in Materials and Methods. (B) Mice received intraperitoneal injections with the indicated amounts of the PIX-blocking or control peptides. They were treated with aerosolized LPS or saline for 6 h and then leakage of Evans blue dye (EB) into the lung assayed as described in Materials and Methods. Values are means ± SD, n = 4–8. *p < 0.02, **p < .001, relative to LPS-treated mice without peptides.
Figure 9.
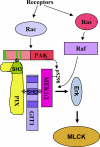
Model for PAK induction of vascular permeability. PAK is activated in response to inflammatory, thrombotic, and angiogenic stimuli. Rac mediates activation of PAK, whereas Ras mediates activation of MEK and Erk. An atypical proline-rich sequence in PAK binds to the βPIX SH3 domain. PIX binds to GIT1 through a region in the C terminus of PIX and the SHD of GIT1. The SHD also binds MEK1/2. PAK phosphorylates MEK on ser298, which facilitates activation of MEK by Raf. Subsequent activation of Erk by MEK leads to activation of MLCK and contractility, which disrupts intercellular junctions.
Similar articles
- p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase.
Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, Lee HY. Lee J, et al. Exp Cell Res. 2005 Jul 15;307(2):315-28. doi: 10.1016/j.yexcr.2005.03.028. Exp Cell Res. 2005. PMID: 15893751 - A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC.
Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. Zhang H, et al. J Neurosci. 2005 Mar 30;25(13):3379-88. doi: 10.1523/JNEUROSCI.3553-04.2005. J Neurosci. 2005. PMID: 15800193 Free PMC article. - Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics.
Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Nayal A, et al. J Cell Biol. 2006 May 22;173(4):587-9. doi: 10.1083/jcb.200509075. J Cell Biol. 2006. PMID: 16717130 Free PMC article. - Pak to the future.
Bagrodia S, Cerione RA. Bagrodia S, et al. Trends Cell Biol. 1999 Sep;9(9):350-5. doi: 10.1016/s0962-8924(99)01618-9. Trends Cell Biol. 1999. PMID: 10461188 Review. - Pak GITs to Aurora-A.
Cotteret S, Chernoff J. Cotteret S, et al. Dev Cell. 2005 Nov;9(5):573-4. doi: 10.1016/j.devcel.2005.10.005. Dev Cell. 2005. PMID: 16256730 Review.
Cited by
- p21-Activated kinase (Pak) regulates airway smooth muscle contraction by regulating paxillin complexes that mediate actin polymerization.
Zhang W, Huang Y, Gunst SJ. Zhang W, et al. J Physiol. 2016 Sep 1;594(17):4879-900. doi: 10.1113/JP272132. Epub 2016 May 29. J Physiol. 2016. PMID: 27038336 Free PMC article. - Impaired spine formation and learning in GPCR kinase 2 interacting protein-1 (GIT1) knockout mice.
Menon P, Deane R, Sagare A, Lane SM, Zarcone TJ, O'Dell MR, Yan C, Zlokovic BV, Berk BC. Menon P, et al. Brain Res. 2010 Mar 4;1317:218-26. doi: 10.1016/j.brainres.2009.11.084. Epub 2010 Jan 4. Brain Res. 2010. PMID: 20043896 Free PMC article. - Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins.
Oh D, Han S, Seo J, Lee JR, Choi J, Groffen J, Kim K, Cho YS, Choi HS, Shin H, Woo J, Won H, Park SK, Kim SY, Jo J, Whitcomb DJ, Cho K, Kim H, Bae YC, Heisterkamp N, Choi SY, Kim E. Oh D, et al. J Neurosci. 2010 Oct 20;30(42):14134-44. doi: 10.1523/JNEUROSCI.1711-10.2010. J Neurosci. 2010. PMID: 20962234 Free PMC article. - Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration.
Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Aghajanian A, et al. J Thromb Haemost. 2008 Sep;6(9):1453-60. doi: 10.1111/j.1538-7836.2008.03087.x. Epub 2008 Jul 19. J Thromb Haemost. 2008. PMID: 18647230 Free PMC article. Review. - The PIX-GIT complex: a G protein signaling cassette in control of cell shape.
Frank SR, Hansen SH. Frank SR, et al. Semin Cell Dev Biol. 2008 Jun;19(3):234-44. doi: 10.1016/j.semcdb.2008.01.002. Epub 2008 Jan 20. Semin Cell Dev Biol. 2008. PMID: 18299239 Free PMC article. Review.
References
- Bagrodia S., Bailey D., Lenard Z., Hart M., Guan J. L., Premont R. T., Taylor S. J., Cerione R. A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 1999;274:22393–22400. - PubMed
- Bannerman D. D., Goldblum S. E. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab. Invest. 1999;79:1181–1199. - PubMed
- Beeser A., Jaffer Z. M., Hofmann C., Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 2005;280:36609–36615. - PubMed
- Bokoch G. M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. - PubMed
- Chew T. L., Masaracchia R. A., Goeckeler Z. M., Wysolmerski R. B. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J. Muscle Res. Cell Motil. 1998;19:839–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL75092/HL/NHLBI NIH HHS/United States
- HL73361/HL/NHLBI NIH HHS/United States
- T32 HL007284/HL/NHLBI NIH HHS/United States
- P01 HL073361/HL/NHLBI NIH HHS/United States
- R01 HL075092/HL/NHLBI NIH HHS/United States
- 5T32 HL7284-27/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous