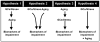A new glucocorticoid hypothesis of brain aging: implications for Alzheimer's disease - PubMed (original) (raw)
Review
A new glucocorticoid hypothesis of brain aging: implications for Alzheimer's disease
Philip W Landfield et al. Curr Alzheimer Res. 2007 Apr.
Abstract
The original glucocorticoid (GC) hypothesis of brain aging and Alzheimer's disease proposed that chronic exposure to GCs promotes hippocampal aging and AD. This proposition arose from a study correlating increasing plasma corticosterone with hippocampal astrocyte reactivity in aging rats. Numerous subsequent studies have found evidence consistent with this hypothesis, in animal models and in humans. However, several results emerged that were inconsistent with the hypothesis, highlighting the need for a more definitive test with a broader panel of biomarkers. We used microarray analyses to identify a panel of hippocampal gene expression changes that were aging-dependent, and also corticosterone-dependent. These data enabled us to test a key prediction of the GC hypothesis, namely, that the expression of most target biomarkers of brain aging should be regulated in the same direction (increased or decreased) by both GCs and aging. This prediction was decisively contradicted, as a majority of biomarker genes were regulated in opposite directions by aging and GCs, particularly inflammatory and astrocyte-specific genes. Thus, the initial hypothesis of simple positive cooperativity between GCs and aging must be rejected. Instead, our microarray data suggest that in the brain GCs and aging interact in more complex ways that depend on the cell type. Therefore, we propose a new version of the GC-brain aging hypothesis; its main premise is that aging selectively increases GC efficacy in some cell types (e.g., neurons), enhancing catabolic processes, whereas aging selectively decreases GC efficacy in other cell types (e.g., astrocytes), weakening GC anti-inflammatory activity. We also propose that changes in GC efficacy might be mediated in part by cell type specific shifts in the antagonistic balance between GC and insulin actions, which may be of relevance for Alzheimer's disease pathogenesis.
Figures
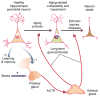
Figure 1. Initial glucocorticoid (GC) hypothesis of hippocampal aging
Long-term exposure to adrenal GCs/stress hormones (red arrows) facilitates aging-related functional decline and vulnerability in hippocampal neurons. Affected cells show heightened sensitivity to extrinsic injuries and disease, causing hippocampal deterioration and eventual cognitive decline. Not shown are activated astrocytes which presumably react to the neuronal changes. See discussion in text. (Redrawn from [2] with permission.)
Figure 2. Possible variations on the interaction between aging and glucocorticoids
Four sub-hypotheses on the mode of positive cooperativity between aging and GCs. See discussion in text. (Redrawn from [2] with permission.)
Figure 3. Overlap of aging-related and corticosterone (Cort)-sensitive genes
Using microarray technology, the genes that were aging-sensitive (upper) and also Cort- sensitive (lower) were identified. Numbers shown represent the number of genes in each category of directional change (down or up). Most genes downregulated with aging are expressed primarily in neurons, whereas upregulated genes are primarily glial. More genes were altered in opposite directions by Cort and aging than in the same direction, contravening a key prediction of the original GC hypothesis. Selected processes (and some genes from which they were identified) are shown for each directional combination of Cort and aging changes.
Figure 4. New GC hypothesis of hippocampal aging
Model incorporating aging-dependent decreases as well as increases in GC efficacy, in different cell type-specific compartments. Target processes (from Fig. (3)) altered during aging by strengthening Cort efficacy (left) should shift with aging in the direction promoted by GCs, whereas targets altered by weakening Cort efficacy (right) should shift with aging in the direction opposite to that normally promoted by GCs. Therefore, in target cells where Cort and aging effects are in same direction (left), Cort efficacy is proposed to increase with aging, and in cells where Cort and aging effects are in opposite directions (right), Cort efficacy is proposed to decrease. Target processes that are regulated by either increased or decreased Cort efficacy may be normally suppressed (−) or activated (+) by GCs. Putative neuronal or glial loci of the effects are based on direction (down or up) of change with aging.
Similar articles
- Glucocorticoid-dependent hippocampal transcriptome in male rats: pathway-specific alterations with aging.
Chen KC, Blalock EM, Curran-Rauhut MA, Kadish I, Blalock SJ, Brewer L, Porter NM, Landfield PW. Chen KC, et al. Endocrinology. 2013 Aug;154(8):2807-20. doi: 10.1210/en.2013-1139. Epub 2013 Jun 4. Endocrinology. 2013. PMID: 23736296 Free PMC article. - Mechanisms of neuronal death in brain aging and Alzheimer's disease: role of endocrine-mediated calcium dyshomeostasis.
Landfield PW, Thibault O, Mazzanti ML, Porter NM, Kerr DS. Landfield PW, et al. J Neurobiol. 1992 Nov;23(9):1247-60. doi: 10.1002/neu.480230914. J Neurobiol. 1992. PMID: 1469387 Review. - Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models.
Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Norris CM, et al. J Neurosci. 2005 May 4;25(18):4649-58. doi: 10.1523/JNEUROSCI.0365-05.2005. J Neurosci. 2005. PMID: 15872113 Free PMC article. - Nathan Shock Memorial Lecture 1990. The role of glucocorticoids in brain aging and Alzheimer's disease: an integrative physiological hypothesis.
Landfield PW. Landfield PW. Exp Gerontol. 1994 Jan-Feb;29(1):3-11. doi: 10.1016/0531-5565(94)90058-2. Exp Gerontol. 1994. PMID: 8187839 Review. - Glucocorticoid receptors modulate dendritic spine plasticity and microglia activity in an animal model of Alzheimer's disease.
Pedrazzoli M, Losurdo M, Paolone G, Medelin M, Jaupaj L, Cisterna B, Slanzi A, Malatesta M, Coco S, Buffelli M. Pedrazzoli M, et al. Neurobiol Dis. 2019 Dec;132:104568. doi: 10.1016/j.nbd.2019.104568. Epub 2019 Aug 5. Neurobiol Dis. 2019. PMID: 31394203
Cited by
- Altered expression of claudin family proteins in Alzheimer's disease and vascular dementia brains.
Romanitan MO, Popescu BO, Spulber S, Băjenaru O, Popescu LM, Winblad B, Bogdanovic N. Romanitan MO, et al. J Cell Mol Med. 2010 May;14(5):1088-100. doi: 10.1111/j.1582-4934.2009.00999.x. J Cell Mol Med. 2010. PMID: 20041969 Free PMC article. - Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient Alzheimer's disease.
Blalock EM, Buechel HM, Popovic J, Geddes JW, Landfield PW. Blalock EM, et al. J Chem Neuroanat. 2011 Oct;42(2):118-26. doi: 10.1016/j.jchemneu.2011.06.007. Epub 2011 Jul 2. J Chem Neuroanat. 2011. PMID: 21756998 Free PMC article. - Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex.
Patel PD, Katz M, Karssen AM, Lyons DM. Patel PD, et al. Psychoneuroendocrinology. 2008 Apr;33(3):360-7. doi: 10.1016/j.psyneuen.2007.12.003. Epub 2008 Jan 28. Psychoneuroendocrinology. 2008. PMID: 18222612 Free PMC article. - Aged rats are hypo-responsive to acute restraint: implications for psychosocial stress in aging.
Buechel HM, Popovic J, Staggs K, Anderson KL, Thibault O, Blalock EM. Buechel HM, et al. Front Aging Neurosci. 2014 Feb 12;6:13. doi: 10.3389/fnagi.2014.00013. eCollection 2014. Front Aging Neurosci. 2014. PMID: 24575039 Free PMC article. - FK506-binding protein 1b/12.6: a key to aging-related hippocampal Ca2+ dysregulation?
Gant JC, Blalock EM, Chen KC, Kadish I, Porter NM, Norris CM, Thibault O, Landfield PW. Gant JC, et al. Eur J Pharmacol. 2014 Sep 15;739:74-82. doi: 10.1016/j.ejphar.2013.10.070. Epub 2013 Nov 28. Eur J Pharmacol. 2014. PMID: 24291098 Free PMC article. Review.
References
- Landfield PW. An endocrine hypothesis of brain aging and studies on brain-endocrine correlations and monosynaptic neurophysiology during aging. Adv Exp Med Biol. 1978;113:179–99. - PubMed
- Porter NM, Landfield PW. Stress hormones and brain aging: adding injury to insult? Nat Neurosci. 1998 May;1(1):3–4. - PubMed
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978 Dec 8;202(4372):1098–102. - PubMed
- Feldman S, Conforti N. Feedback effects of dexamethasone on adrenocortical responses in rats with fornix section. Horm Res. 1976;7(1):56–60. - PubMed
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991 May;12(2):118–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous