Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP - PubMed (original) (raw)
Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP
Shahana Baig-Lewis et al. Dev Biol. 2007.
Abstract
Members of the Wg/Wnt family provide key intercellular signals during embryonic development and in the maintenance of homeostatic processes, but critical aspects of their signal transduction pathways remain controversial. We have found that canonical Wg signaling in Drosophila involves distinct initiation and amplification steps, both of which require Arrow/LRP. Expressing a chimeric Frizzled2-Arrow protein in flies that lack endogenous Wg or Arrow showed that this construct functions as an activated Wg receptor but is deficient in signal amplification. In contrast, a chimeric Arrow protein containing the dimerization domain of Torso acted as a potent amplifier of Wg signaling but could not initiate Wg signaling on its own. The two chimeric proteins synergized, so that their co-expression largely reconstituted the signaling levels achieved by expressing Wg itself. The amplification function of Arrow/LRP appears to be particularly important for long-range signaling, and may reflect a general mechanism for potentiating signals in the shallow part of a morphogen gradient.
Figures
Figure 1. A graded requirement for Wg signaling in the anterior-posterior axis of developing embryos
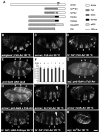
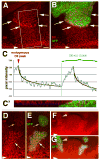
(A) Schematic representation of constructs used in this study. The transmembrane domain (TM) is indicated for Arrow; “*” denotes the Y327C mutation that renders Torso dominant. UAS-Arr is from Wehrli et al., 2000; UAS-Fz2-Arr, UAS-Fz2myc, Fz2-eCFP are from Tolwinski et al. (2003), UAS-torWTArr and torDArr are described in this study. (B–M) In all of these panels, prd-Gal4 was used to drive construct expression, while the temperature-dependence of Gal4 activity was used to vary expression levels. (B–D, I–M) dark-field illumination of embryonic cuticles. (B) In a _wingless_null mutant embryo, driving expression of Fz2-Arr with prd-Gal4 generates four bands of smooth cuticle, signifying a full rescue. (C) Using the same expression system in an arrownull embryo, only a partial rescue is observed. (D) In a _fz_− _fz2_− embryo, Fz2-Arr expression provides full rescue. (E) prd-Gal4 driven UAS-lacZ in a stage 10 embryo (30 °C) is visualized by immunostaining and detected by confocal microscopy. The posterior four bands of prd-Gal4 expression (numbered 1–4) in the abdominal region are responsible for generating the smooth cuticle bands induced by Fz2-Arr expression (shown in (B) and (D); also numbered 1–4). All stripes of lacZ expression show similar intensities. (F) prd-Gal4 driven lacZ expression in stage 10 embryos (see panel E) was quantified as average fluorescence within a stripe. Average fluorescence between corresponding stripes of different embryos was then compared (error bars indicate standard deviations). No graded expression is detected in the anterioposterior direction. (G) prd-Gal4 driven Fz2-Arr expression (30 °C) visualized by immunofluorescence in a wgnull embryo, using the 6xmyc tag present in Fz2-Arr (detected with anti-myc antibodies). All stripes express similar levels of Fz2-Arr, which are statistically indistinguishable from expression levels seen in arrnull mutants (H, and see text). (H) arrnull mutant embryo expressing Fz2-Arr with prd-Gal4 (30°C) and visualized as in (G). Similar levels of Fz2-Arr expression are detected in the stripes of these animals, which are comparable to expression levels detected in wgnull embryos (see text). (I) Expression of two copies of Fz2-Arr generates 3 bands of abdominal smooth cuticle (#3 is incompletely separated); a very narrow and incomplete 4th band of smooth cuticle is also visible (arrowhead). Rescue is clearly enhanced by the higher dosage of Fz2-Arr (compare with C). (J) Limiting the expression of UAS-Arr in an arrnull mutant embryo (28°C) results in a partial rescue (an anterior versus posterior difference is apparent; compare with the rescue patterns seen in (C, I). (K) Limiting the expression of Fz2myc in a _fz_− _fz2_− embryo shows also loss of rescue in the posterior rather then anterior region of the embryo (similar to (J). (L) Partial rescue of _fz_− _fz2_− embryo with expression of Fz2-Arr is apparent from the incomplete formation of smooth cuticle band #3 (arrowhead), but occurs only when expression levels are severely reduced (at 22 °C), compared to Fz2 expression at 28 °C (K). (M) Maximal expression of torDArr in _wg_− induces no smooth cuticle. Bar = 40μm in B–E, G–M. Data in panels B & C were previously presented in Tolwinski et al., 2003 and are included here for comparison.
Figure 2
torDArr potentiates Wg signaling in the embryo and expands smooth cuticle fate. (A) In wild type embryos (wt), six rows of denticles differentiate with characteristic denticle orientation and shapes. (B) torDArr expressed with ptc-Gal4 causes the loss of several rows of posterior denticles (rows 3–6). (C) Expression of Fz2-Arr under the control of ptc-Gal4 re-specifies these posterior denticles as smooth cuticle (data from Tolwinski et al., 2003). In (A–C), only part of the smooth cuticle territory is shown. (D) Schematic diagram showing the expression domains for Ptc and Wg in two adjacent segments of embryonic cuticle (modified from Hatini and DiNardo (2001)). Ptc is induced by Hedgehog, which is produced by Engrailed (En) cells; Wg ligand is rapidly degraded in En cells resulting in an asymmetric Wg gradient (Dubois et al., 2001). Bar = 10 μm in A–C.
Figure 3
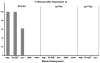
torDArr activity requires an existing Wg signal. As summarized in the left panel, Fz2-Arr expressed in the prd-Gal4 pattern fully rescues smooth cuticle in the absence of either Wg or the Frizzleds (_fz_− _fz2_−), and substantially rescues smooth cuticle in the absence of Arrow. However, Fz2-Arr signaling critically depends on Dishevelled and Armadillo (data shown previously in Tolwinski et al., 2003). In contrast, expression of torDArr expressed in the prd-Gal4 pattern fails to rescue smooth cuticle in the absence of any of the Wg pathway components tested (middle panel). Mutants tested and number of embryos (rescued/not rescued) are as follows: _wg_CX4 0/163; _fz_H51 _fz2_C1 0/137; arr2 0/258; _dsh_V26 0/283; _arm_XM19 0/88. Expression of torWTArr with prd-Gal4 also fails to generate smooth cuticle (right panel); _wg_CX4 0/172; _fz_H51 _fz2_C1 0/72; arr2 0/67. (P<10−4 or better in χ2 analysis; in these experiments, 25% of embryos are of the genotype that could potentially display rescue).
Figure 4
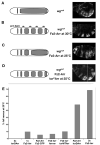
Fz2-Arr and torDArr synergize to restore Wg signaling in the embryo. (A–D) show schematic diagrams and representative preparations of each manipulation. (A) A winglessnull mutant is devoid of smooth cuticle. (B) In winglessnull mutants raised at 30°C (to maximize Gal4-activity), prd-Gal4 drives expression of Fz2-Arr in alternate segments, providing maximal rescue (see also Tolwinski et al. (2003)). (C) When these animals are raised at 25°C to reduce Gal4 transcriptional activity and Fz2-Arr levels, incomplete rescue ensues. (D) Introducing torDArr in such an embryo restores full rescue. Such embryos are frequently curled; the right panel shows two focal planes to reveal all four abdominal smooth cuticle bands. (E) Quantification of the ability of different combinations of our chimeric constructs to potentiate signaling and fully rescue the genotype shown in (C). No signaling was detected with 2x torDArr alone (see also Fig. 2); 1x Fz2-Arr results in full rescue 10% of the time (4/40), and no significant increase is detected by co-expression of Fz2-Arr with Fz2-CFP (5/88), Arrow (2/29), or torWTArr (8/77) (P<0.0001). A significant increase in signaling is only observed upon co-expression of torDArr (17/35) or a second copy of Fz2-Arr (36/52) (P<0.0001).
Figure 5
torDArr greatly potentiates Wg signaling. Maximal expression UAS constructs in the wing was accomplished using the MS1096-Gal4 driver at 30°C. (A) wild type wing, anterior is up. (B) Over-expression of Arrow (UAS-Arrow) induces ectopic margin bristles; ectopic posterior hairs are indicated by black arrows (Wehrli et al., 2000). (C, C′) Few ectopic margin bristles (arrows) are induced by torWTArr expression (UAS-torWTArr), and minor venation defects occur near the margin. C′ shows magnified view of boxed region in C. (D, D′) High level expression of torDArr (UAS-torDArr) covers most of the wing in bristles and hairs. The bristles and hairs are appropriate for each sector of the wing, which is reflected by the distributions of different types of ectopic bristles: stout triple row bristles are seen near the anterior margin (black arrow), slender double row bristles are seen in the medial-distal sector (arrowhead) and fine hairs are seen posteriorly (white arrow). Because the unequal expression of torDArr (driven by MS1096-Gal4) causes distortions in the wing and diminishes wing size, no wing veins are visible in this preparation. In (D′) some ectopic stout triple row bristles and slender double row bristles are indicated with arrows and arrowheads, respectively. (E, E′) torDArr at 25°C only induces bristles in a band adjacent to the wing margin, demonstrating its dependence on endogenous Wg that originates at the margin (brackets). In (E′) stout bristles (arrows) and slender bristles (arrowheads) are distinguishable. As in (D), the wing is distorted due to unequal growth of dorsal and ventral wing blades. To account for these distortions, images for (D, D′, E, & E′) were collected as bright-field Z stacks, optically flattened, and assembled as mosaics (see Material and methods for details). In order to allow for viability of flies shown in Fig. 6B, the wing in Fig. 5 E, E′ is from a fly containing two copies of torDArr but that, like wings in Fig. 6B–F, is heterozygous for the MS1096-Gal4 driver and has half the dose of Wingless (MS1096-Gal4/+; _wg_−/+; 2x UAS-torDArr; see Material and methods for details). Bar = 50 μm in (A, C); 25 μm in (D, E); 12.5 μm in (B, C′, D′, E′).
Figure 6
Synergy between torDArr and Fz2-Arr approximates the normal signal transduction levels of Wg ligand. In (A–F), attenuated Wg signaling at 25°C permits flies of even the most severe phenotype (e.g. panel C) to survive (see Material and methods). (A, A′) bristles induced by Fz2-Arr expression are predominantly near the margin (arrows) but are also found closer to the center of the wing blade (inset); venation defects are apparent (arrowheads), predominantly around vein III–V. In (A′) ectopic stout triple row bristles and slender double row bristles are indicated with arrows and arrowheads, respectively. Also visible, due to the high contrast, are the much smaller trichomes (‘wing hairs’) produced by each cell in the wing, as in wild type. (B, B′) The synergistic effects of co-expressing torDArr with Fz2-Arr produce wings that are covered in margin bristles. In (B′) many ectopic stout triple row bristles (arrows) and slender double row bristles (arrowheads) are visible. Thrichomes are visible in the area not covered by large bristles, as in wild type. (C) Fz2-Arr alone displays only minor venation defects (arrowhead). (D) Co-expression of torWTArr with Fz2-Arr induces minor venation defects (arrowhead) and some ectopic bristles (arrows). (E) Co-expression of Arrow with Fz2-Arr causes minor venation defects (arrowhead) and ectopic bristles (arrow). (F) Co-expression of Fz2-CFP with Fz2-Arr generates ectopic bristles (arrows) and causes loss of wing veins; as in Figs. 5D–E, 6B, B′) unequal growth between dorsal and ventral wing blades distorts the wing. (G, H) Co-expression of torDArr and Fz2-Arr re-constitutes normal Arrow function in the Wg signaling pathway. (G) At 18°C, the levels of Wg ligand over-expression (UAS-Wg) induced by the MS1096-Gal4 driver are sufficiently low to permit flies to complete development. However, this level of Wg expression still induced a band of ectopic margin bristles (arrow); arrowhead indicates the anterior wing margin (the wing is still partly folded). (H) Co-expression of torDArr and Fz2-Arr induces a similar number of ectopic rows of bristles as the Wg ligand itself (compare with G). In (G), the wing was mounted; in (H), the wing was directly photographed to minimize distortions (see Material and methods). The higher resolution images shown in panels A′ and B′ were generated as described in Fig. 5. Bar = 50 μm for (A, C–F), (A′, B) 25 μm, (B′) 12.5 μm, (G, H) 32 μm.
Figure 7. torDArr functions cell-autonomously
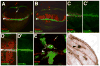
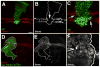
In all confocal micrographs, dorsal is up, and white arrowheads mark the D/V boundary. Larvae were reared at 30°C to induce maximal torDArr expression levels. (A) Wg enhancer trap expression (Wg-lacZ, green) along the dorsoventral (D/V) compartment boundary (the future wing margin, white arrowhead) is straddled by expression of the Wg target gene Senseless (Sense, red) in sensory organ precursor cells (SOP). (B) Expression of torDArr in the wing pouch with MS1096-Gal4 induces many additional Senseless-positive cells (red), correlating with future bristle cells. Wg-lacZ expression is unchanged by torDArr expression. (C) Extracellular Wg is visualized using a protocol that allows immunostaining of secreted Wg (C′ green; as in Strigini and Cohen (2000)), demonstrating the spread of Wg ligand away from the source at the D/V boundary, which is flanked by Senseless expressing cells (red; compare to (A)). (D) Expression of torDArr induces many ectopic Senseless expressing cells (red), suggesting maximal transduction of the Wg signal, yet no increase in extracellular Wg is apparent. (D′) shows the Wg channel alone. (E) Both the endogenous expression pattern of Senseless and the ectopic Senseless cells are visible in the same tissue, but all ectopic Senseless-positive cells are confined to clones expressing torDArr (GFP-positive, green). Note that clones need not be directly adjacent to Wg-expressing cells to be capable of inducing Senseless expression (yellow arrows). The endogenous pattern of Senseless expression can be seen flanking the D/V boundary (arrowhead) is apparent, plus a large Senseless-positive cell resides in the center of the presumptive wing blade (arrow, compare to wild type, in (A)). (F) The expression of torDArr in clones is manifested as tufts of bristles in the adult wing. Several clones are shown containing either stout bristles of the anterior triple row type (arrowhead) or slender bristles of double row type (arrows). Note that these clones form ‘islands’ that are not connected to the wing margin, which is the endogenous source of Wg (as noted above). Bar = 50 μm in (A, B), 25 μm (C, D), 10 μm (E), 8μm (F)
Figure 8. torDArr amplifies the Wg signal in response to available ligand
For A–C, larvae were reared at 30°C, while for D–G, larvae were reared at 25°C to maintain lower torDArr expression levels. (A, B) torDArr-expressing clones (green) also exhibit a cell-autonomous increase in the expression of the Wg target gene Distal-less (Dll, red). Within the clone, Dll expression diminishes in a graded fashion away from the source of Wg at the D/V boundary (indicated by arrows), reflecting the Wg-dependence of signal potentiation by torDArr. (C) The endogenous Dll gradient profile matches the Dll gradient generated in a clone expressing torDArr. In order to accommodate the three-dimensional nature of this tissue, Dll staining in the region indicated in (A) was determined as and the graph indicates expression levels as a function of the distance from the endogenous signal peak (at the D/V boundary, arrowhead in A; see Material and methods). As apparent in panel A, a sharply defined edge of high-level expression is visible at the border of the clone facing the D/V boundary. The gradual increase of Dll expression levels reflects averaging across the curved boundary of the clone and the slanted angle of the epithelium. The shape of the gradient fits a logarithmic curve. (C′) A vertical section reveals that tissue folds position the GFP expressing clone at an angle similar to the Dll profile observed in (C) and explains why the Dll increase appears gradual. This vertical section through the Z stack along the middle of the box shown in (A); green, GFP; red, Dll; the blue horizontal line marks the level of the section shown in (A, B). (D, E) A torDArr-expressing clone (green, white arrow) near the D/V boundary (arrowhead; the source of Wg) expresses higher levels of Dll than a more distant clone (yellow arrow), illustrating that the level of signal amplification by torDArr is proportional to the level of Wg. (F, G) Lower magnification of a Z stack projection of the preparation containing the clones shown in (D, E). An additional elongated clone (yellow arrowhead) shows a graded decline with distance from the Wg source, though adjacent tissue expresses considerably lower levels of Dll. Bar = 17 μm in (A, B), 10 μm in (D, E), 20 μm (F, G).
Figure 9
Induction of Senseless expression by Fz2-Arr + torDArr in _wg_− clones. (A–C) A _wg_− clone (green) intersects the endogenous Senseless (red) stripe flanking the endogenous Wg expressing cells at the D/V boundary. Wg from surrounding wild type tissue can diffuse and rescue adjacent Senseless expression in _wg_− cells (yellow arrows in C), while the remainder of the clone fails to express Senseless (white arrow in B). (B) shows a schematic projection of the clone boundary in A. (C) shows a higher magnification of (A). (D–F) _wg_− clone (green) expressing Fz2-Arr + torDArr induces Senseless expression across the entire clone (region between yellow arrowheads in F), extending well beyond the single row of cells at the clone boundary seen in (C). The clone distorts the wing disc and has its narrowest point at the D/V boundary (between arrowheads); panels D–F show a projection of a partial Z stack to accommodate this distortion. Bar = 10 μm in A, B), 4 μm (C), 30 μm (D, E), 4.2 μm (F).
Figure 10
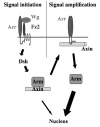
A two-step model for Wg signal transduction. The Wg receptor complex consisting of Arrow and Frizzleds initiates the cytoplasmic cascade in response to Wg binding. Dishevelled transmits this response to the Axin complex and may destabilize the complex sufficiently to transduce a minor signal to the nucleus, via free Armadillo. For maximal signaling, Axin relocates to the cell membrane, where it interacts with clustered Arrow/LRP and becomes inactivated. Armadillo may then be released from Axin and newly synthesized Armadillo is no longer destroyed, resulting in amplified transcriptional activation in the nucleus.
Similar articles
- Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors.
Schweizer L, Varmus H. Schweizer L, et al. BMC Cell Biol. 2003 May 2;4:4. doi: 10.1186/1471-2121-4-4. BMC Cell Biol. 2003. PMID: 12729465 Free PMC article. - Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing.
Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Rives AF, et al. Dev Biol. 2006 May 1;293(1):268-83. doi: 10.1016/j.ydbio.2006.02.006. Epub 2006 Mar 10. Dev Biol. 2006. PMID: 16530179 Free PMC article. - Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc.
Han C, Yan D, Belenkaya TY, Lin X. Han C, et al. Development. 2005 Feb;132(4):667-79. doi: 10.1242/dev.01636. Epub 2005 Jan 12. Development. 2005. PMID: 15647319 - Wnt/Wingless signaling in Drosophila.
Swarup S, Verheyen EM. Swarup S, et al. Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a007930. doi: 10.1101/cshperspect.a007930. Cold Spring Harb Perspect Biol. 2012. PMID: 22535229 Free PMC article. Review. - Cellular mechanisms of wingless/Wnt signal transduction.
Dierick H, Bejsovec A. Dierick H, et al. Curr Top Dev Biol. 1999;43:153-90. doi: 10.1016/s0070-2153(08)60381-6. Curr Top Dev Biol. 1999. PMID: 9891886 Review.
Cited by
- Wnt/beta-catenin signaling: new (and old) players and new insights.
Huang H, He X. Huang H, et al. Curr Opin Cell Biol. 2008 Apr;20(2):119-25. doi: 10.1016/j.ceb.2008.01.009. Epub 2008 Mar 12. Curr Opin Cell Biol. 2008. PMID: 18339531 Free PMC article. Review. - Wnt/beta-catenin signaling: components, mechanisms, and diseases.
MacDonald BT, Tamai K, He X. MacDonald BT, et al. Dev Cell. 2009 Jul;17(1):9-26. doi: 10.1016/j.devcel.2009.06.016. Dev Cell. 2009. PMID: 19619488 Free PMC article. Review. - Destruction complex dynamics: Wnt/β-catenin signaling alters Axin-GSK3β interactions in vivo.
Lybrand DB, Naiman M, Laumann JM, Boardman M, Petshow S, Hansen K, Scott G, Wehrli M. Lybrand DB, et al. Development. 2019 Jul 2;146(13):dev164145. doi: 10.1242/dev.164145. Development. 2019. PMID: 31189665 Free PMC article. - Histone lysine methyltransferase Pr-set7/SETD8 promotes neural stem cell reactivation.
Huang J, Gujar MR, Deng Q, Y Chia S, Li S, Tan P, Sung WK, Wang H. Huang J, et al. EMBO Rep. 2021 Apr 7;22(4):e50994. doi: 10.15252/embr.202050994. Epub 2021 Feb 10. EMBO Rep. 2021. PMID: 33565211 Free PMC article. - The way Wnt works: components and mechanism.
Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E. Saito-Diaz K, et al. Growth Factors. 2013 Feb;31(1):1-31. doi: 10.3109/08977194.2012.752737. Epub 2012 Dec 21. Growth Factors. 2013. PMID: 23256519 Free PMC article. Review.
References
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–1832. - PubMed
- Babu P. Early developmental subdivisions of the wing disk in Drosophila. Mol Gen Genet. 1977;151:289–294. - PubMed
- Barnea E, Sorkin R, Ziv T, Beer I, Admon A. Evaluation of prefractionation methods as a preparatory step for multidimensional based chromatography of serum proteins. Proteomics. 2005;5:3367–3375. - PubMed
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM067029/GM/NIGMS NIH HHS/United States
- GM67029/GM/NIGMS NIH HHS/United States
- P01-GM067166/GM/NIGMS NIH HHS/United States
- P01 GM067166/GM/NIGMS NIH HHS/United States
- GM045747/GM/NIGMS NIH HHS/United States
- R01 GM045747/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous