Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function - PubMed (original) (raw)
Review
Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function
Leslie P Henderson. Neuropharmacology. 2007 Jun.
Abstract
The hypothalamus, the seat of neuroendocrine control, is exquisitely sensitive to gonadal steroids. For decades it has been known that androgens, estrogens and progestins, acting through nuclear hormone receptors, elicit both organizational and activational effects in the hypothalamus and basal forebrain that are essential for reproductive function. While changes in gene expression mediated by these classical hormone pathways are paramount in governing both sexual differentiation and the neural control of reproduction, it is also clear that steroids impart critical control of neuroendocrine functions through non-genomic mechanisms. Specifically, endogenous neurosteroid derivatives of deoxycorticosterone, progesterone and testosterone, as well and synthetic anabolic androgenic steroids that are self-administered as drugs of abuse, elicit acute effects via allosteric modulation of gamma-aminobutyric acid type A receptors. GABAergic transmission within the hypothalamus and basal forebrain is a key regulator of pubertal onset, the expression of sexual behaviors, pregnancy and parturition. Summarized here are the known actions of steroid modulators on GABAergic transmission within the hypothalamus/basal forebrain, with a focus on the medial preoptic area and the supraoptic/paraventricular nuclei that are known to be central players in the control of reproduction.
Figures
Figure 1
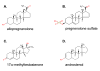
Representative steroid modulators of the GABAA receptor for A) the positive neurosteroids: allopregnanolone, b) the negative neurosteroids: pregnenolone sulfate, C) the AAS: 17α-methyltestosterone and D) pheromones: androstenol. Structures are from the ChemIDplus website:
http://chem.sis.nlm.nih.gov/chemidplus/
.
Figure 2. Anatomical correlates of female reproductive behaviors
Schematic representation of a midline-sagittal section and hypothalamic/basal forebrain or downstream midbrain regions in which GABAergic transmission facilitates (green) or antagonize (red) lordosis in rodents. mPOA: medial preoptic nucleus, VMN: ventromedial nucleus of the hypothalamus, VTA: ventral tegmental area, MCG: midbrain central gray; cc: corpus callosum.
Figure 3
(A) Levels of ε subunit mRNA in the mPOA as assessed by standard semi-quantitative RT-PCR. Data are normalized to values from adult, gonadally-intact C57Bl/6 males (100%). (B) Percent potentiation in average peak current (Ipeak) over control elicited by 3α-diol.
Figure 4
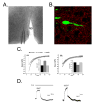
A) Representative micrograph of an acutely isolated slice from a female mouse at the level of the mPOA and a patch electrode. B) Micrograph of the mPOA processed for synaptophysin immunocytochemistry (red) from the transgenic line of mice in which GFP is expressed in GnRH neurons (green) amidst a number of non-GnRH cell bodies (black). Mice were kindly provided by Dr. Sue Moenter (University of Virginia). C) Representative sIPSCs recorded from an identified GFP-GnRH neuron demonstrating reversible potentiation (left) and inhibition (right) of peak current amplitude by 1 μM 17α-MeT. D) Representative whole-cell currents recorded in the absence of GABA from an acutely dissociated GFP-GnRH neuron. Left: current arising from spontaneously active GABAA receptors. No exogenous GABA was applied; spontaneous current is antagonized by the noncompetitive GABAA receptor antagonist, picrotoxin. Right: spontaneous current from the same GFP-GnRH neuron demonstrating reversible inhibition by a maximally effective (10 μM) concentration of 17α-MeT. V3: Third Ventricle. Data are courtesy of Dr. Carlos Penatti, Dr. Brian Jones and Ms. Sandra Pahl.
Similar articles
- Anabolic androgenic steroids and forebrain GABAergic transmission.
Henderson LP, Penatti CA, Jones BL, Yang P, Clark AS. Henderson LP, et al. Neuroscience. 2006;138(3):793-9. doi: 10.1016/j.neuroscience.2005.08.039. Epub 2005 Nov 28. Neuroscience. 2006. PMID: 16310317 Review. - Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain.
Oberlander JG, Porter DM, Penatti CA, Henderson LP. Oberlander JG, et al. J Neuroendocrinol. 2012 Jan;24(1):202-14. doi: 10.1111/j.1365-2826.2011.02151.x. J Neuroendocrinol. 2012. PMID: 21554430 Free PMC article. Review. - Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions.
Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CA, Porter DM, Yang P, Henderson LP. Clark AS, et al. Brain Res. 2006 Dec 18;1126(1):122-38. doi: 10.1016/j.brainres.2006.08.081. Epub 2006 Sep 29. Brain Res. 2006. PMID: 17010954 Review. - Neurosteroid modulation of GABAA receptors.
Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Lambert JJ, et al. Prog Neurobiol. 2003 Sep;71(1):67-80. doi: 10.1016/j.pneurobio.2003.09.001. Prog Neurobiol. 2003. PMID: 14611869 Review. - The function and the expression of forebrain GABA(A) receptors change with hormonal state in the adult mouse.
Jorge JC, McIntyre KL, Henderson LP. Jorge JC, et al. J Neurobiol. 2002 Feb 5;50(2):137-49. doi: 10.1002/neu.10021. J Neurobiol. 2002. PMID: 11793360
Cited by
- Neurotoxicity by synthetic androgen steroids: oxidative stress, apoptosis, and neuropathology: A review.
Pomara C, Neri M, Bello S, Fiore C, Riezzo I, Turillazzi E. Pomara C, et al. Curr Neuropharmacol. 2015 Jan;13(1):132-45. doi: 10.2174/1570159X13666141210221434. Curr Neuropharmacol. 2015. PMID: 26074748 Free PMC article. Review. - Pheromones, binding proteins, and olfactory systems in the pig (Sus scrofa): An updated review.
Sankarganesh D, Kirkwood RN, Nagnan-Le Meillour P, Angayarkanni J, Achiraman S, Archunan G. Sankarganesh D, et al. Front Vet Sci. 2022 Dec 1;9:989409. doi: 10.3389/fvets.2022.989409. eCollection 2022. Front Vet Sci. 2022. PMID: 36532348 Free PMC article. Review. - Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse.
Costine BA, Oberlander JG, Davis MC, Penatti CA, Porter DM, Leaton RN, Henderson LP. Costine BA, et al. Psychoneuroendocrinology. 2010 Nov;35(10):1473-85. doi: 10.1016/j.psyneuen.2010.04.015. Epub 2010 May 26. Psychoneuroendocrinology. 2010. PMID: 20537804 Free PMC article. - Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse.
Penatti CA, Oberlander JG, Davis MC, Porter DM, Henderson LP. Penatti CA, et al. Neuropharmacology. 2011 Sep;61(4):653-64. doi: 10.1016/j.neuropharm.2011.05.008. Epub 2011 May 27. Neuropharmacology. 2011. PMID: 21645530 Free PMC article. - Effects of chronic exposure to an anabolic androgenic steroid cocktail on alpha5-receptor-mediated GABAergic transmission and neural signaling in the forebrain of female mice.
Penatti CA, Costine BA, Porter DM, Henderson LP. Penatti CA, et al. Neuroscience. 2009 Jun 30;161(2):526-37. doi: 10.1016/j.neuroscience.2009.03.039. Epub 2009 Mar 24. Neuroscience. 2009. PMID: 19324077 Free PMC article.
References
- Araki T, Kiyama H, Tohyama M. The GABAA receptor γ1 subunit is expressed by distinct neuronal populations. Molecular Brain Research. 1992;15:121–132. - PubMed
- Araki T, Kiyama H, Maeno H, Tohyama M. Differential immunocytochemical localization of GABAA receptor γ1 and γ2 subunits in the rat brain. Molecular Brain Research. 1993;20:263–266. - PubMed
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87. - PubMed
- Baum MJ. Mammalian animal models of psychosexual differentiation: When it ‘translation’ to the human situation possible? Hormones and Behavior. 2006;50:579–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA018255/DA/NIDA NIH HHS/United States
- R01 DA014137/DA/NIDA NIH HHS/United States
- R01 DA014137-09A1/DA/NIDA NIH HHS/United States
- R01 DA018255-03/DA/NIDA NIH HHS/United States
- DA/NS14137/DA/NIDA NIH HHS/United States
- DA18255/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources