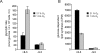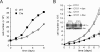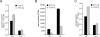The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis - PubMed (original) (raw)
The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis
Julian J Lum et al. Genes Dev. 2007.
Abstract
Mammalian cells are believed to have a cell-intrinsic ability to increase glucose metabolism in response to hypoxia. Here we show that the ability of hematopoietic cells to up-regulate anaerobic glycolysis in response to hypoxia is dependent on receptor-mediated signal transduction. In the absence of growth factor signaling, hematopoietic cells fail to express hypoxia-inducible transcription factor (Hif-1alpha) mRNA. Growth factor-deprived hematopoietic cells do not engage in glucose-dependent anabolic synthesis and neither express Hif-1alpha mRNA nor require HIF-1alpha protein to regulate cell survival in response to hypoxia. However, HIF-1alpha is adaptive for the survival of growth factor-stimulated cells, as suppression of HIF-1alpha results in death when growing cells are exposed to hypoxia. Growth factor-dependent HIF-1alpha expression reprograms the intracellular fate of glucose, resulting in decreased glucose-dependent anabolic synthesis and increased lactate production, an effect that is enhanced when HIF-1alpha protein is stabilized by hypoxia. Together, these data suggest that HIF-1alpha contributes to the regulation of growth factor-stimulated glucose metabolism even in the absence of hypoxia.
Figures
Figure 1.
Induction of glycolysis by hypoxia is dependent on growth factor signals. (A) Cells were cultured in the presence or absence (3 wk) of IL-3. Following 24 h incubation at 21% O2 or 1.5% O2, cellular glucose utilization was measured by the conversion of 5-3H-glucose to 3H2O. Data represent mean of three independent experiments ± SD. (B) Glucose-dependent lipid synthesis was measured by labeling cells with D-[U-14C6] glucose for 24 h. Lipid extracts were obtained from 2 × 106 cells as described in the Materials and Methods. Data are a representative experiment performed in triplicate ± SD.
Figure 2.
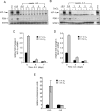
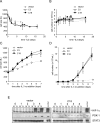
Growth factors regulate the expression and activity of HIF-1α. (A) Cells cultured in the presence (+IL3) or absence of IL-3 at the indicated time points were incubated in normoxic (N) or hypoxic (H) conditions for 4 h and HIF-1α protein levels were analyzed by Western blot. Cells cultured in the presence of IL-3 and treated with 100 μM CoCl2 were used as a control. (B) At similar time points following withdrawal from IL-3, cells were treated with PBS or 100 μM CoCl2 for 4 h and analyzed by Western blot for HIF-1α and PDK-1 protein levels. Lysates from cells cultured in the presence of IL-3 exposed to hypoxia were used as a control. STAT3 was used as a loading control. Cells were cultured in the presence or absence of IL-3, and at the indicated time points, total RNA was isolated and analyzed for _Hif-1_α (C) or Glut1 (D) mRNA levels by real-time qPCR. Data are a representative experiment for triplicate samples ± SD. (E) HIF-1α promoter activity in response to hypoxia is lost during growth factor withdrawal. HIF-1α-responsive promoter containing three tandem HRE from the murine pgk1 was transfected into cells cultured in the presence or absence (3 wk) of IL-3. Ten hours after transfection, cells were incubated in normoxic (21% O2) or hypoxic (1.5% O2) conditions for an additional 24 h. Promoter activity is expressed as a ratio of luciferase to Renilla activity (relative luminescence units). Data are a representative experiment ± SD. for triplicate samples.
Figure 3.
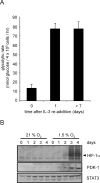
Control of glycolytic recovery following growth factor restimulation does not involve HIF-1α expression. (A) Glycolytic rate of cells cultured in the absence of growth factor for 4 wk was measured at the indicated time points following readdition of IL-3. Graph is a representative experiment plotted as the mean ± SD of one triplicate experiment. (B) At various time points following IL-3 readdition, cells were subjected to normoxic or hypoxic conditions for an additional 4 h. Cell lysates were collected and Western blot analysis was used to measure HIF-1α and PDK-1 protein levels. STAT3 was used as a loading control.
Figure 4.
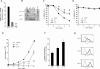
Cells expressing stable shRNA targeting HIF-1α have impaired survival but enhanced proliferative capacity. (A) Real-time qPCR analysis of _Hif-1_α mRNA levels. RNA from cells was isolated and cDNA synthesis was performed as described in the Materials and Methods section. Data are a representative experiment. (B) Western blotting of HIF-1α and PDK-1 in clones stably expressing shRNA to HIF-1α after culturing for 4 h in normoxic (N) or hypoxic (H) conditions. Lysates from vector cells cultured in the presence of IL-3 and exposed to 100 μM CoCl2 were used as a positive control. (C) Viability of HIF-1α knockdown cells grown in the presence of IL-3 cultured under hypoxic condition. At each time point, cells were collected and viable cells were assessed by staining with propidium iodide. Data represent mean of three independent experiments ± SD. (D) Viability of HIF-1α knockdown cells grown in the absence of IL-3 cultured under hypoxic condition. At each time point, cells were collected and viable cells assessed by staining with propidium iodide. Data represent mean of three independent experiments ± SD. (E) Growth curves of IL-3-stimulated cells expressing stable HIF-1α knockdown cultured under normoxic conditions. Data are a representative experiment performed in triplicate ± SD. (F) The rate of oxygen consumption under normoxia was measured in HIF-1α knockdown cells cultured in the presence of IL-3. Data are a representative experiment performed in triplicate ± SD. (G) Mitochondrial membrane potential was measured in cells cultured in the presence of IL-3 using the potentiometric dye TMRE. The value in the top right corner is a representative value of the mean channel fluorescence (MCF). Unstained cells and stained cells are represented by light and bold histograms, respectively.
Figure 5.
Cellular proliferation is controlled by the levels of HIF-1α. (A) Growth curves of primary T cells isolated from Hif-1αfl/fl Cre− (wild-type [WT]) or Hif-1fl/fl Cre+ (Cre) mice that were administered tamoxifen. Two independent experiments were performed and the data presented are one representative experiment. (B) Population doublings (PD) of NIH3T3 cells expressing an inducible form of a constitutively activated HIF-1α. Two independent clones were cultured in the presence or absence of doxycycline. Data are a representative experiment performed in triplicate ± SD. Inset shows a Western blot of HIF-1α expression in the transfected cells. Cells were treated with 200 μM desferrioxamine (cntrl), or exposed to vehicle control (−), or 0.5 μg/mL doxycycline (+). The doxycycline induction of transfected HIF-1α is comparable to the level of induction of endogenous HIF-1α in response to desferrioxamine. Total Akt was used as a loading control.
Figure 6.
Cell growth and proliferation are enhanced in the absence of HIF-1α. Cells were withdrawn from IL-3 and size (A) and cell number (B) were measured at the indicated time points. On day 16 of withdrawal, cells were restimulated with IL-3 and size (C) and cell number (D) were measured. Data represent the mean of three independent experiments ± SD. (E) Cells cultured in the absence of IL-3 for 16 d were placed in fresh complete medium containing IL-3. At the various time points, cells were subjected to an additional 4 h of culture under 21% O2 (N) or 1.5% O2 (H). Cell lysates were analyzed by Western blot for HIF-1α and PDK-1 expression. A representative vector control and a HIF-1α cell line (C18) expressing a stable HIF-1α shRNA are shown. STAT3 was used as a loading control. Cells cultured in the presence of IL-3 and exposed to hypoxia were used as a positive control.
Figure 7.
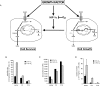
Induction of glycolysis through HIF-1α is anti-proliferative. (A) Growth factors stimulate cellular glucose uptake and the expression of HIF-1α. Under conditions of normoxia, the accumulation of HIF-1α is repressed by O2-dependent hydroxylation and degradation of the HIF-1α protein. When HIF-1α is repressed by O2-dependent degradation and/or HIF-1α shRNA, glycolytic pyruvate is diverted into mitochondrial-dependent lipid synthesis. Pyruvate is catabolized by mitochondrial PDH into acetyl-CoA, which is used in the TCA cycle to produce citrate that is transported into the cytosol, where it acts as a further regulator of glucose-6-P metabolism. Citrate is metabolized to produce acetyl-CoA by the enzyme ATP-citrate lyase (ACL) to supply the cell with a source of acetyl-CoA for lipid synthesis. When HIF-1α is stabilized by mitochondrial ROS or oxygen deprivation, the increased HIF-1α-dependent transcription can result in a metabolic reprogramming of intracellular glucose fate. The HIF-1α-dependent increases in glycolytic genes including Ldh-A lead to an enhanced rate of anaerobic glycolysis, and the induction of mitochondrial regulatory enzymes such as PDK-1 decrease pyruvate metabolism in the mitochondria, resulting in decreased mitochondrial TCA cycle activity and cytosolic citrate levels. These effects would support non-oxygen-dependent ATP production by degradation of glucose to lactate, and suppress mitochondrial activity and cytosolic lipid synthesis. (B) Glycolytic rate of cells cultured in the presence of IL-3 following 24 h incubation under 21% O2 or 1.5% O2. In the absence of IL-3 (not shown), glycolysis was reduced <6.58 nmol of glucose per hour in both the vector controls and HIF-1α shRNA cells. Data are the mean of three independent experiments ± SD performed in triplicate. (C) Cells cultured in the presence of IL-3 were subjected to 4 h of 21% O2 or 1.5% O2 followed by an additional 20–24 h incubation with 14C-labeled pyruvate. Lipids were extracted from cell lysates and total incorporation of 14C-labeled lipid was measured by scintillation counting. Data are a representative experiment ± SD for triplicate samples. (D) Accumulation of lactate in cell culture supernatants. Cells were cultured in 1 mL of medium for 5 d in the presence of IL-3 and subjected to 1.5% O2 for an additional 24 h. Lactate levels in supernatants were measured as described in Materials and Methods. Data represent three independent experiments ± SD.
Figure 8.
Glucose utilization in primary T cells is impaired by HIF-1α. (A) Glucose consumption in primary T cells isolated from Hif-1αfl/fl Cre− (wild-type [WT]) or Hif-1fl/fl Cre+ (Cre) mice that were administered tamoxifen. T cells were stimulated under 21% or 1.5% oxygen by plate-bound anti-CD3/anti-CD28 in 1-mL cell cultures, and supernatants were collected 48 h post-stimulation for glucose analysis. Data are a representative experiment performed in triplicate ± SD. (B) Cells were activated by plate-bound anti-CD3/ anti-CD28 for 2 d followed by addition of 14C-labeled pyruvate under normoxic or hypoxic conditions. Cells were cultured for an additional 2 d in the presence of IL-2. Lipids were extracted from cell lysates and total incorporation of 14C-labeled lipid was measured by scintillation counting. Data are a representative experiment ± SD for triplicate samples. (C) Cells were cultured under identical conditions described in A and the amount of secreted lactate into 1 mL of culture medium was measured as described in Materials and Methods. Data are a representative experiment ± SD.
Similar articles
- Hypoxia-induced overexpression of DEC1 is regulated by HIF-1α in hepatocellular carcinoma.
Ma W, Shi X, Lu S, Wu L, Wang Y. Ma W, et al. Oncol Rep. 2013 Dec;30(6):2957-62. doi: 10.3892/or.2013.2774. Epub 2013 Oct 1. Oncol Rep. 2013. PMID: 24100543 Retracted. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Functional importance of Dicer protein in the adaptive cellular response to hypoxia.
Ho JJ, Metcalf JL, Yan MS, Turgeon PJ, Wang JJ, Chalsev M, Petruzziello-Pellegrini TN, Tsui AK, He JZ, Dhamko H, Man HS, Robb GB, Teh BT, Ohh M, Marsden PA. Ho JJ, et al. J Biol Chem. 2012 Aug 17;287(34):29003-20. doi: 10.1074/jbc.M112.373365. Epub 2012 Jun 28. J Biol Chem. 2012. PMID: 22745131 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Adipose Tissue Hypoxia in Obesity: Clinical Reappraisal of Hypoxia Hypothesis.
Engin A. Engin A. Adv Exp Med Biol. 2024;1460:329-356. doi: 10.1007/978-3-031-63657-8_11. Adv Exp Med Biol. 2024. PMID: 39287857 Review.
Cited by
- Ubiquitin regulatory X (UBX) domain-containing protein 6 is essential for autophagy induction and inflammation control in macrophages.
Kim YJ, Lee SG, Park SY, Jeon SM, Kim SI, Kim KT, Roh T, Lee SH, Lee MJ, Lee J, Kim HJ, Lee SE, Kim JK, Heo JY, Kim IS, Park C, Paik S, Jo EK. Kim YJ, et al. Cell Mol Immunol. 2024 Dec;21(12):1441-1458. doi: 10.1038/s41423-024-01222-1. Epub 2024 Oct 23. Cell Mol Immunol. 2024. PMID: 39438692 Free PMC article. - Activation of Epidermal Growth Factor Receptor/p38/Hypoxia-inducible Factor-1α Is Pivotal for Angiogenesis and Tumorigenesis of Malignantly Transformed Cells Induced by Hexavalent Chromium.
Kim D, Dai J, Park YH, Fai LY, Wang L, Pratheeshkumar P, Son YO, Kondo K, Xu M, Luo J, Shi X, Zhang Z. Kim D, et al. J Biol Chem. 2016 Jul 29;291(31):16271-81. doi: 10.1074/jbc.M116.715797. Epub 2016 May 25. J Biol Chem. 2016. PMID: 27226640 Free PMC article. Retracted. - Definition of a Novel Feed-Forward Mechanism for Glycolysis-HIF1α Signaling in Hypoxic Tumors Highlights Aldolase A as a Therapeutic Target.
Grandjean G, de Jong PR, James B, Koh MY, Lemos R, Kingston J, Aleshin A, Bankston LA, Miller CP, Cho EJ, Edupuganti R, Devkota A, Stancu G, Liddington RC, Dalby K, Powis G. Grandjean G, et al. Cancer Res. 2016 Jul 15;76(14):4259-4269. doi: 10.1158/0008-5472.CAN-16-0401. Epub 2016 Jun 3. Cancer Res. 2016. PMID: 27261507 Free PMC article. - Dual-modality, dual-functional nanoprobes for cellular and molecular imaging.
Menon JU, Gulaka PK, McKay MA, Geethanath S, Liu L, Kodibagkar VD. Menon JU, et al. Theranostics. 2012;2(12):1199-207. doi: 10.7150/thno.4812. Epub 2012 Dec 31. Theranostics. 2012. PMID: 23382776 Free PMC article. - Succinate dehydrogenase 5 regulates lung cancer metastasis by reprogramming glucose metabolism.
Li J, Tuo Z, Zong Y, Liu J. Li J, et al. J Thorac Dis. 2021 Nov;13(11):6427-6438. doi: 10.21037/jtd-21-1769. J Thorac Dis. 2021. PMID: 34992822 Free PMC article.
References
- Arsham A.M., Plas D.R., Thompson C.B., Simon M.C., Plas D.R., Thompson C.B., Simon M.C., Thompson C.B., Simon M.C., Simon M.C. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 α nor sufficient for HIF-1-dependent target gene transcription. J. Biol. Chem. 2002;277:15162–15170. - PubMed
- Bauer D.E., Harris M.H., Plas D.R., Lum J.J., Hammerman P.S., Rathmell J.C., Riley J.L., Thompson C.B., Harris M.H., Plas D.R., Lum J.J., Hammerman P.S., Rathmell J.C., Riley J.L., Thompson C.B., Plas D.R., Lum J.J., Hammerman P.S., Rathmell J.C., Riley J.L., Thompson C.B., Lum J.J., Hammerman P.S., Rathmell J.C., Riley J.L., Thompson C.B., Hammerman P.S., Rathmell J.C., Riley J.L., Thompson C.B., Rathmell J.C., Riley J.L., Thompson C.B., Riley J.L., Thompson C.B., Thompson C.B. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J. 2004;18:1303–1305. - PMC - PubMed
- Bentley J., Itchayanan D., Barnes K., McIntosh E., Tang X., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Itchayanan D., Barnes K., McIntosh E., Tang X., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Barnes K., McIntosh E., Tang X., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., McIntosh E., Tang X., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Tang X., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Downes C.P., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Holman G.D., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Whetton A.D., Owen-Lynch P.J., Baldwin S.A., Owen-Lynch P.J., Baldwin S.A., Baldwin S.A. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J. Biol. Chem. 2003;278:39337–39348. - PubMed
- Blum R., Jacob-Hirsch J., Amariglio N., Rechavi G., Kloog Y., Jacob-Hirsch J., Amariglio N., Rechavi G., Kloog Y., Amariglio N., Rechavi G., Kloog Y., Rechavi G., Kloog Y., Kloog Y. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Res. 2005;65:999–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases