Soluble epoxide hydrolase: a novel therapeutic target in stroke - PubMed (original) (raw)
Soluble epoxide hydrolase: a novel therapeutic target in stroke
Wenri Zhang et al. J Cereb Blood Flow Metab. 2007 Dec.
Abstract
The P450 eicosanoids epoxyeicosatrienoic acids (EETs) are produced in brain and perform important biological functions, including protection from ischemic injury. The beneficial effect of EETs, however, is limited by their metabolism via soluble epoxide hydrolase (sEH). We tested the hypothesis that sEH inhibition is protective against ischemic brain damage in vivo by a mechanism linked to enhanced cerebral blood flow (CBF). We determined expression and distribution of sEH immunoreactivity (IR) in brain, and examined the effect of sEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE) on CBF and infarct size after experimental stroke in mice. Mice were administered a single intraperitoneal injection of AUDA-BE (10 mg/kg) or vehicle at 30 mins before 2-h middle cerebral artery occlusion (MCAO) or at reperfusion, in the presence and absence of P450 epoxygenase inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH). Immunoreactivity for sEH was detected in vascular and non-vascular brain compartments, with predominant expression in neuronal cell bodies and processes. 12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester was detected in plasma and brain for up to 24 h after intraperitoneal injection, which was associated with inhibition of sEH activity in brain tissue. Finally, AUDA-BE significantly reduced infarct size at 24 h after MCAO, which was prevented by MS-PPOH. However, regional CBF rates measured by iodoantipyrine (IAP) autoradiography at end ischemia revealed no differences between AUDA-BE- and vehicle-treated mice. The findings suggest that sEH inhibition is protective against ischemic injury by non-vascular mechanisms, and that sEH may serve as a therapeutic target in stroke.
Figures
Figure 1
Soluble epoxide hydrolase (sEH) expression in brain. Western blot analysis of brain vascular and non-vascular compartments shows that sEH is predominantly expressed in brain’s parenchymal, and to a lesser degree in cerebral vessels. Vascular smooth muscle α-actin is restricted to the vessel fraction, suggesting effective separation of both compartments. Western blot is representative of three blots.
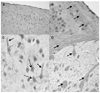
Figure 2
Localization of soluble epoxide hydrolase (sEH) immunoreactivity in mouse brain neuronal cell bodies and processes. Immunoreactivity for sEH in the cerebral cortex (A to C) and striatum (D) is localized to neuronal cell bodies (dotted arrows) and processes (solid arrows). Three immunohistochemistry runs were performed on n = 3 mice.
Figure 3
Pharmacokinetic profile of sEH inhibitor AUDA-BE and its metabolites in plasma. AUDA-BE was administered to mice as a single intraperitoneal injection of either 40 mg/kg (A) or 10 mg/kg (B). 12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester and the metabolites AUDA and AUBA were analyzed at 1, 3, 6, and 24 h after injection. Control injections of sesame oil without AUDA-BE led to concentrations of parent drug or metabolites below the detection limits (0.96, 1.10, and 5.78 nmol/L for AUDA, AUDA-BE, and AUBA, respectively). Control values were subtracted from corresponding values at each time point. AUBA is a biologically inactive indicator metabolite. The mean value at each time point represents two animals.
Figure 4
12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester inhibits soluble epoxide hydrolase enzymatic activity in mouse brain tissue after systemic administration. Activity was measured at 1, 3, 6, and 24 h after single AUDA-BE administration (10 mg/kg intraperitoneally) using sEH-specific surrogate substrate [3H]-_trans_-1,3-diphenylpropene oxide (tDPPO). Average activity over 24 h was reduced from 1.92 ± 0.06nmol/mg in vehicle-treated mice to 1.56 ± 0.13 nmol/mg protein in AUDA-BE-treated mice (n = 2) at each of four time points: 1, 3, 6, and 24 h after injection, P < 0.05.
Figure 5
12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester decreases infarct size after middle cerebral artery occlusion (MCAO) in mice. Infarct size was reduced from 33 ± 2% (n = 5) in vehicle-treated mice to 20±5% (n = 10) and 16±6% (n = 5) when AUDA-BE was administered (10 mg/kg intraperitoneally) 1 h before (pre) or at the time of reperfusion (post) after 2-h MCAO. *Different from vehicle (P<0.05).
Figure 6
P450 epoxygenase inhibitor _N_-methylsulfonyl-6-(2-propargylloxyphenyl) hexanamide (MS-PPOH) eliminates protection by AUDA-BE. Infract size was reduced by AUDA-BE alone (10 mg/kg, a single intraperitoneal injection at reperfusion) from 33±2% to 16±6% (n = 5 per group). However, when combined with MS-PPOH (0.5 mg/200 µL over 24 h before MCAO, via subcutaneously implanted osmotic mini-pumps), AUDA-BE loses it protective effect (infarct size 34±7%, n = 5, not different from vehicle). *Different from vehicle (P<0.05).
Figure 7
Blood flow rates distribution in mouse brain during MCAO. Flow rates were quantified by [14C]-iodoantipyrine (IAP) autoradiography at the end of 2-h MCAO in mice treated with vehicle (sesame oil, n = 5) or AUDA-BE (10 mg/kg intraperitoneally, 30 mins before MCAO, n = 5). (A) Color-coded distribution of regional CBF rates. (B) Blood flow distribution in the ipsilateral hemisphere. No differences were observed in the amount of tissue (mm3) perfused with any given flow rate (mL/100 g per min) between vehicle- and AUDABE-treated mice (n = 5 per group).
Similar articles
- Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo.
Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Motoki A, et al. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2128-34. doi: 10.1152/ajpheart.00428.2008. Epub 2008 Oct 3. Am J Physiol Heart Circ Physiol. 2008. PMID: 18835921 Free PMC article. - Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia.
Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Zhang W, et al. Stroke. 2008 Jul;39(7):2073-8. doi: 10.1161/STROKEAHA.107.508325. Epub 2008 Mar 27. Stroke. 2008. PMID: 18369166 Free PMC article. - An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats.
Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. Dorrance AM, et al. J Cardiovasc Pharmacol. 2005 Dec;46(6):842-8. doi: 10.1097/01.fjc.0000189600.74157.6d. J Cardiovasc Pharmacol. 2005. PMID: 16306811 Free PMC article. - Soluble epoxide hydrolase: a new target for cardioprotection.
Gross GJ, Nithipatikom K. Gross GJ, et al. Curr Opin Investig Drugs. 2009 Mar;10(3):253-8. Curr Opin Investig Drugs. 2009. PMID: 19333883 Free PMC article. Review. - Soluble epoxide hydrolase inhibitors and heart failure.
Qiu H, Li N, Liu JY, Harris TR, Hammock BD, Chiamvimonvat N. Qiu H, et al. Cardiovasc Ther. 2011 Apr;29(2):99-111. doi: 10.1111/j.1755-5922.2010.00150.x. Cardiovasc Ther. 2011. PMID: 20433684 Free PMC article. Review.
Cited by
- Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets.
Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, Hu J, Fleming I, Wang DW. Wang B, et al. Signal Transduct Target Ther. 2021 Feb 26;6(1):94. doi: 10.1038/s41392-020-00443-w. Signal Transduct Target Ther. 2021. PMID: 33637672 Free PMC article. Review. - Peroxisomal translocation of soluble epoxide hydrolase protects against ischemic stroke injury.
Nelson JW, Zhang W, Alkayed NJ, Koerner IP. Nelson JW, et al. J Cereb Blood Flow Metab. 2015 Sep;35(9):1416-20. doi: 10.1038/jcbfm.2015.159. Epub 2015 Jul 1. J Cereb Blood Flow Metab. 2015. PMID: 26126869 Free PMC article. - Soluble epoxide hydrolase inhibition provides multi-target therapeutic effects in rats after spinal cord injury.
Chen X, Chen X, Huang X, Qin C, Fang Y, Liu Y, Zhang G, Pan D, Wang W, Xie M. Chen X, et al. Mol Neurobiol. 2016 Apr;53(3):1565-1578. doi: 10.1007/s12035-015-9118-1. Epub 2015 Feb 10. Mol Neurobiol. 2016. PMID: 25663200 - Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population.
Zhang L, Ding H, Yan J, Hui R, Wang W, Kissling GE, Zeldin DC, Wang DW. Zhang L, et al. Pharmacogenet Genomics. 2008 Jan;18(1):45-51. doi: 10.1097/FPC.0b013e3282f313e8. Pharmacogenet Genomics. 2008. PMID: 18216721 Free PMC article. - Arachidonic acid cytochrome P450 epoxygenase pathway.
Spector AA. Spector AA. J Lipid Res. 2009 Apr;50 Suppl(Suppl):S52-6. doi: 10.1194/jlr.R800038-JLR200. Epub 2008 Oct 23. J Lipid Res. 2009. PMID: 18952572 Free PMC article. Review.
References
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996a;271:H1541–H1546. - PubMed
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. - PubMed
- Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PD. Neuroprotection and P4502C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–1684. - PubMed
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996b;27:971–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 ES002710-27/ES/NIEHS NIH HHS/United States
- ES02710/ES/NIEHS NIH HHS/United States
- P01 NS049210/NS/NINDS NIH HHS/United States
- R01 HL059699/HL/NHLBI NIH HHS/United States
- R01 NS044313/NS/NINDS NIH HHS/United States
- NS049210/NS/NINDS NIH HHS/United States
- NS44313/NS/NINDS NIH HHS/United States
- ES004699/ES/NIEHS NIH HHS/United States
- R01 ES002710/ES/NIEHS NIH HHS/United States
- P42 ES004699-21/ES/NIEHS NIH HHS/United States
- GM31278/GM/NIGMS NIH HHS/United States
- P42 ES004699/ES/NIEHS NIH HHS/United States
- R01 GM031278/GM/NIGMS NIH HHS/United States
- HL59699-06A1/HL/NHLBI NIH HHS/United States
- R37 ES002710/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials