Incentives and disincentives to participation by clinicians in randomised controlled trials - PubMed (original) (raw)
Review
Incentives and disincentives to participation by clinicians in randomised controlled trials
J M Rendell et al. Cochrane Database Syst Rev. 2007.
Abstract
Background: Patients and clinicians need reliable, up-to-date information from randomised controlled trials (RCTs) on the costs and benefits of treatments. Recruitment difficulties arise when clinicians do not invite patients to participate in trials.
Objectives: Primary: to assess the evidence for the effect of disincentives and incentives on the extent to which clinicians invite eligible patients to participate in RCTs of healthcare interventions. Secondary: to assess the evidence in relation to stated willingness to invite participation.
Search strategy: 1. The Cochrane Methodology Register and Cochrane Database of Methodology Reviews were searched in May 2006 and Cochrane Central Register of Controlled Trials, National Research Register and ClinicalTrialsGov in April 2005.2. EMBASE, MEDLINE, CINAHL, PsycINFO and AMED were searched in April 2005.3. Reference lists of included studies were checked.
Selection criteria: Studies exploring the effect of (dis)incentives on clinicians' views and recruitment-related activity.
Data collection and analysis: The information about included studies was insufficient for a full assessment of quality. Data on (dis)incentives were extracted and association with recruitment tested.
Main results: No RCTs of interventions were identified. Eleven observational studies were included - two medical records reviews, one matched pair study, one clinician interview study, two studies documenting clinicians' decisions and five postal surveys. Three measures of recruitment were used, invitation to participate, entry into RCT and reported entry to RCT. Five studies explored the effect of patient characteristics. The effect of age and prognosis varied between trials. Six studies considered the association between clinicians' views and recruitment. Clinicians who agreed to participate because they were acquainted with the researchers were less likely to participate than those otherwise motivated (1 study, 2-sided p = 0.04 Fisher's exact test) and (Odds Ratio [OR] 0.4, 95% Confidence Interval [CI] 0.2 to 0.9, 1 study). Clinicians who had recruited were more likely to report some difficulties including "trials involve extra work" (OR 92.94, 95% CI 4.54 - 1902.11; p </= 0.01, 1 study) and "inviting patients to participate is embarrassing" (chi-square 15.55, df = 1, p < 0.0001, 1 study). The effect of the need to discuss clinical uncertainty was unclear but concern that the doctor-patient relationship would be adversely affected by participation was a deterrent (chi-square = 7.25, df = 1, p = 0.007, 1 study).
Authors' conclusions: The impact of factors varied across studies. Researchers need to be aware that aspects of the design and conduct of trials can affect clinicians' willingness to invite patients to participate. Further research is needed.
Conflict of interest statement
Jennifer Rendell, John Geddes and Rowena Merritt are not aware of any potential conflicts of interest.
Figures
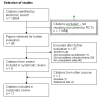
1
Selection of studies.
1.1. Analysis
Comparison 1 Age of participant, Outcome 1 Invited to participate.
Update of
- doi: 10.1002/14651858.MR000021.pub2
Similar articles
- The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - Strategies to improve recruitment to randomised controlled trials.
Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, Sullivan F, Wilson S, Jackson C, Jones R, Mitchell E. Treweek S, et al. Cochrane Database Syst Rev. 2010 Apr 14;(4):MR000013. doi: 10.1002/14651858.MR000013.pub5. Cochrane Database Syst Rev. 2010. PMID: 20393971 Updated. Review. - Incentives for preventing smoking in children and adolescents.
Hefler M, Liberato SC, Thomas DP. Hefler M, et al. Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article. Review. - Strategies to improve recruitment to randomised controlled trials.
Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, Sullivan F, Wilson S, Jackson C, Jones R. Treweek S, et al. Cochrane Database Syst Rev. 2010 Jan 20;(1):MR000013. doi: 10.1002/14651858.MR000013.pub4. Cochrane Database Syst Rev. 2010. PMID: 20091668 Updated. Review. - Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.
Marshall M, Crowther R, Almaraz-Serrano A, Creed F, Sledge W, Kluiter H, Roberts C, Hill E, Wiersma D, Bond GR, Huxley P, Tyrer P. Marshall M, et al. Health Technol Assess. 2001;5(21):1-75. doi: 10.3310/hta5210. Health Technol Assess. 2001. PMID: 11532238 Review.
Cited by
- Recruitment and enrollment of participants in an online diabetes self-management intervention in a virtual environment.
Vorderstrasse A, Reagan L, D'Eramo Melkus G, Nowlin SY, Birdsall SB, Burd A, Cho YH, Jang M, Johnson C. Vorderstrasse A, et al. Contemp Clin Trials. 2021 Jun;105:106399. doi: 10.1016/j.cct.2021.106399. Epub 2021 Apr 20. Contemp Clin Trials. 2021. PMID: 33857681 Free PMC article. Clinical Trial. - The advance of research governance in psychiatry: one step forward, two steps back.
Leeson VC, Tyrer P. Leeson VC, et al. Epidemiol Psychiatr Sci. 2013 Dec;22(4):313-20. doi: 10.1017/S2045796013000255. Epub 2013 May 29. Epidemiol Psychiatr Sci. 2013. PMID: 23714283 Free PMC article. - Challenges in reaching patients with severe mental illness for trials in general practice-a convergent mixed methods study based on the SOFIA pilot trial.
Tranberg K, Due TD, Rozing M, Jønsson ABR, Kousgaard MB, Møller A. Tranberg K, et al. Pilot Feasibility Stud. 2023 Oct 31;9(1):182. doi: 10.1186/s40814-023-01395-y. Pilot Feasibility Stud. 2023. PMID: 37908003 Free PMC article. - A thematic analysis of factors influencing recruitment to maternal and perinatal trials.
Tooher RL, Middleton PF, Crowther CA. Tooher RL, et al. BMC Pregnancy Childbirth. 2008 Aug 7;8:36. doi: 10.1186/1471-2393-8-36. BMC Pregnancy Childbirth. 2008. PMID: 18687110 Free PMC article. Review. - Maximising recruitment and retention of general practices in clinical trials: a case study.
Dormandy E, Kavalier F, Logan J, Harris H, Ishmael N, Marteau TM; SHIFT research team. Dormandy E, et al. Br J Gen Pract. 2008 Nov;58(556):759-66, i-ii. doi: 10.3399/bjgp08X319666. Br J Gen Pract. 2008. PMID: 19000399 Free PMC article.
References
References to studies included in this review
Antman 1985 {published data only}
- Antman K, Amato D, Wood W, Carson J, Suit H, Proppe K, Carey R, Greenberger J, Wilson R, Frei E, III. Selection bias in clinical trials. J. Clin. Oncol. 1985;3(8):1142‐1147. - PubMed
de Wit 2001 {published data only}
- Wit NJ, Quartero AO, Zuithoff AP, Numans ME. Participation and successful patient recruitment in primary care. J.Fam.Pract. 2001;50(11):976. - PubMed
Kemeny 2003 {published data only}
- Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, Bartlett N, Fleming G, Cohen HJ. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21(12):2268‐2275. - PubMed
Kuyvenhoven 1997 {published data only}
Lee 1980 {published data only}
- Lee JY, Marks JE, Simpson JR. Recruitment of patients to cooperative group clinical trials. Cancer Clin.Trials 1980;3(4):381‐384. - PubMed
Richardson 2002 {published data only}
- Richardson A, Sutherland M, Wells E, Toop L, Plumridge L. Factors affecting general practitioner involvement in a randomised controlled trial in primary care. New Zealand Medical Journal 2002;115(1151):153‐155. - PubMed
Siminoff 2000 {published data only}
- Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. Journal of Clinical Oncology 2000;18(6):1203‐1211. - PubMed
Simon 1999 {published data only}
- Simon MS, Brown DR, Du W, LoRusso P, Kellogg CM. Accrual to breast cancer clinical trials at a university‐affiliated hospital in metropolitan Detroit. Am J Clin Oncol 1999;22(1):42‐46. - PubMed
Simon 2004 {published data only}
- Simon MS, Du W, Flaherty L, Philip PA, LoRusso P, Miree C, Smith D, Brown DR. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol 2004;22(11):2046‐2052. - PubMed
Taylor 1984 {published data only}
- Taylor KM. The doctor's dilemma: physician participation in randomized clinical trials. Cancer Treatment Reports 1985;69(10):1095‐1100. - PubMed
- Taylor KM, Margolese RG, Soskolne CL. Physicians' reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. New England Journal of Medicine 1984;310(21):1363‐1367. - PubMed
Wilson 2000 {published data only}
- Wilson I, McGrath B, Russell G, Bridges‐Webb C, Hogan C. General practitioners' views on patient care research. Australian Family Physician 2000;29(1):86‐88. - PubMed
References to studies excluded from this review
Hjorth 1996 {published data only}
- Hjorth M, Holmberg E, Rodjer S, Taube A, Westin J. Physicians' attitudes toward clinical trials and their relationship to patient accrual in a Nordic multicenter study on myeloma. Controlled Clinical Trials 1996;17(5):372‐386. - PubMed
Winn 1984 {published data only}
- Winn RJ, Miransky J, Kerner JF, Kennelly L, Michaelson RA, Sturgeon SR. An evaluation of physician determinants in the referral of patients for cancer clinical trials in the community setting. Advances in cancer control: Epidemiology and research 1984;156:62‐73. - PubMed
Additional references
Benjamin 2000
- Benjamin S, Kroll ME, Cartwright RA, Clough JV, Gorst DW, Proctor SJ, Ross JR, Taylor PR, Wheatley K, Whittaker JA, Stiller CA. Haematologists' approaches to the management of adolescents and young adults with acute leukaemia. British Journal of Haematology 2000;111(4):1045‐50. - PubMed
Benson 1991
- Benson AB, III, Pregler JP, Bean JA, Rademaker AW, Eshler B, Anderson K. Oncologists' reluctance to accrue patients onto clinical trials: an Illinois Cancer Center study. Journal of Clinical Oncology 1991;9(11):2067‐2075. - PubMed
Charlson 1984
Collins 1992
- Collins R, Doll R, Peto R. Ethics in clinical trials. In: Williams CJ editor(s). Introducing New Treatments For Cancer: Practical, Ethical and Legal Problems. Chichester: John Wiley, 1992:49‐56.
Costa 2004
- Costa LJ, Xavier AC, Giglio A. Negative results in cancer clinical trials‐equivalence or poor accrual?. Controlled Clinical Trials 2004;25(5):525‐533. - PubMed
CRD Report 4 2001
- Centre for Research and Dissemination, University of York. Undertaking Systematic Reviews of Research on Effectiveness. http://www.york.ac.uk/inst/crd/report4.htm (accessed 28th February 2005).
Easterbrook 1992
Fallowfield 1997
- Fallowfield LJ, Ratcliffe D, Souhami R. Clinicians' Attitudes to Clinical Trials of Cancer Therapy. European Journal of Cancer 1997;33(13):2221‐2229. - PubMed
Guyatt 1995
- Guyatt G, Sackett D, Sinclair J, Hayward R, Cook D, Cook R. Users' Guide to Medical Literature 9. A method for grading health‐care recommendations. JAMA 1995;274:1800‐1804. - PubMed
Klayman 1987
- Klayman J. Confirmation, disconfirmation and information in hypothesis testing.. Psychological Review 1987;94(2):211‐228.
Mapstone 2007
Miller 1975
- Miller DT, Ross M. Self‐serving biases in the attribution of causality: Fact or fiction?. Psychological Bulletin 1975;82:213‐225.
Nickerson 1998
- Nickerson RS. Confirmation bias: A ubiquitous phenomenon in many guises.. Review of general psychology 1998;2:175‐220.
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5:649‐655. - PubMed
Prescott 1999
- Prescott R, Counsell C, Gillespie W, Grant A, Russell I, Kiauka S, Colthart IR, Ross S, Shepherd SM, Russell D. Factors that limit the quality, number and progress of randomised controlled trials. Health Technology Assessment 1999;3(20):1‐139. - PubMed
Sackett 1979
- Sackett DL. Bias in analytical research. Journal of Chronic Diseases 1979;32:51‐63. - PubMed
Shea 1992
- Shea S, Bigger JT, Jr, Campion J, Fleiss JL, Rolnitzky LM, Schron E, Gorkin L, Handshaw K, Kinney MR, Branyon M. Enrollment in clinical trials: institutional factors affecting enrollment in the cardiac arrhythmia suppression trial (CAST). Controlled Clinical Trials 1992;13(6):466‐486. - PubMed
Taylor 1994
- Taylor KM, Feldstein ML, Skeel RT, Pandya KJ, Ng P, Carbone PP. Fundamental dilemmas of the randomized clinical trial process: results of a survey of the 1,737 Eastern Cooperative Oncology Group investigators. Journal of Clinical Oncology 1994;12(9):1796‐1805. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous