Temporal onset of hypoxia and oxidative stress after pulmonary irradiation - PubMed (original) (raw)
Temporal onset of hypoxia and oxidative stress after pulmonary irradiation
Katharina Fleckenstein et al. Int J Radiat Oncol Biol Phys. 2007.
Abstract
Purpose: To investigate the temporal onset of hypoxia following irradiation, and to show how it relates to pulmonary vascular damage, macrophage accumulation, and the production of reactive oxygen species and cytokines. Our previous studies showed that tissue hypoxia in the lung after irradiation contributed to radiation-induced injury.
Methods and materials: Female Fisher 344 rats were irradiated to the right hemithorax with a single dose of 28 Gy. Serial studies were performed up to 20 weeks following irradiation. Radionuclide lung-perfusion studies were performed to detect changes in pulmonary vasculature. Immunohistochemical studies were conducted to study macrophages, tissue hypoxia (carbonic anhydrase-9 marker), oxidative stress (8-hydroxy-2'-deoxyguanosine), and the expression of profibrogenic (transforming growth factor-beta [TGF-beta]) and proangiogenic (vascular endothelial growth factor [VEGF]) cytokines.
Results: Significant changes in lung perfusion along with tissue hypoxia were observed 3 days after irradiation. Significant oxidative stress was detected 1 week after radiation, whereas macrophages started to accumulate at 4 weeks. A significant increase in TGF-beta expression was seen within 1 day after radiation, and for VEGF at 2 weeks after radiation. Levels of hypoxia, oxidative stress, and both cytokines continued to rise with time after irradiation. The steepest increase correlated with vast macrophage accumulation.
Conclusions: Early changes in lung perfusion, among other factors initiate, the development of hypoxia and chronic oxidative stress after irradiation. Tissue hypoxia is associated with a significant increase in the activation of macrophages and their continuous production of reactive oxygen species, stimulating the production of fibrogenic and angiogenic cytokines, and maintaining the development of chronic radiation-induced lung injury.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1
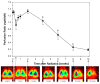
Time related changes in lung perfusion after 28 Gy single dose irradiation to the right hemithorax of rats. Mean perfusion ratio (see Methods) as a function of time (at pre-irradiation time point, T=0, for a period of 10 weeks post-irradiation). A significant decrease in lung perfusion was observed 3 days after irradiation (p=0.0322). After a short recovery period lung perfusion progressively declined from the 2 week post-irradiation time point, until the conclusion. Data are represented as mean (± 1 SEM) observed in 10 animals per time point. *p<0.05 compared with no radiation. Representative scintigraphic images (see Methods) in one animal at pre-irradiation (control) and throughout the 10 week time-course (3 days – 10 weeks) are shown for comparison. Color ramp is used to demonstrate radiolabled perfusion marker with activity levels from low to high indicated by orange < yellow < green < blue < majenta.
Fig. 2
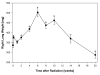
The wet weight of the right lung was recorded immediately after harvesting. At 6 weeks after irradiation the increase in lung weight coincides with edema and inflammation. This is followed by a steady decline in lung weight related to shrinkage and progressive fibrotic changes of the lung. Data are represented as mean obtained from five animals per timepoint, error bars are ± 1 SEM. *p<0.05 compared with no radiation.
Fig. 3
Representative immunohistochemistry staining of the right lung of rats prior to and at different times after irradiation. Brown staining indicates positive staining. Bars represent 100μm.
- activated macrophages (ED-1)
- hypoxia (CA-9)
- ROS (8-OHdG)
- VEGF
- TGF-β
Fig. 4
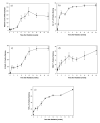
Quantitative analyses from immunohistochemistry images prior to and at different times after irradiation of the right hemithorax with a single dose of 28 Gy. Data are represented as mean obtained from 5 animals per timepoint, error bars are ± 1 SEM. *p<0.05, first significant change compared with no radiation.
- Number of activated macrophages (ED-1): A significant increase in macrophages was seen 4 weeks after irradiation (p=0.0231) and peaked at 10 weeks (p=0.00979).
- Hypoxia (CA-9): A significant increase in CA-9 expression was already seen 3 days after irradiation (p=0.000327) and increased further with time.
- ROS (8-OHdG): A significant increase in 8-OHdG expression started 1 week after irradiation (p=0.0097) and increased further with time.
- VEGF: A significant increase in VEGF expression started 2 weeks after irradiation (p=0.0284).
- TGF-β: A significant increase in TGF-β expression was already seen 1 day after irradiation (p=0.00146) and continuously increased further with time.
Fig. 5
Proposed time related events: Based on the results of this study we hypothezise that early events in radation induced lung injury are based on early vascular changes. This is most likely mediated by several mechanisms: First, an imbalance of ROS and RNS leading to a depletion of nitric oxide (NO˙) due to reaction with superoxide (˙O2-) to peroxynitrite (ONO2-) could cause vasoconstriction as seen in Fig. 1. Initial ˙O2- and ONO2- production activates TGF-β that continuously contributes to radiation induced damage. Second, an enhanced expression of other vasoconstrictive mediators such as Angiotensin II (Ang II) and Endothelin , can also be implicated in this process. Endothelin may also lead to acute pulmonary edema. These early vascular changes contribute to the development of hypoxia, which further enhances oxidative stress, depletion of nitric oxide (NO˙) and expression of HIF-1α and its downstream genes like VEGF and Antiotensin II. The combination of circumstances facilitates the recruitment of inflammatory cells like macrophages, which in turn enhance cytokine and ROS production and further promote hypoxia due to enhanced activation and proliferation maintaining the cycle of repair processes that lead to chronic injury.
Similar articles
- Radiation-induced hypoxia may perpetuate late normal tissue injury.
Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Vujaskovic Z, et al. Int J Radiat Oncol Biol Phys. 2001 Jul 15;50(4):851-5. doi: 10.1016/s0360-3016(01)01593-0. Int J Radiat Oncol Biol Phys. 2001. PMID: 11429211 - Superoxide dismutase mimetic reduces hypoxia-induced O2*-, TGF-beta, and VEGF production by macrophages.
Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Jackson IL, et al. Free Radic Res. 2007 Jan;41(1):8-14. doi: 10.1080/10715760600913150. Free Radic Res. 2007. PMID: 17164174 - Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation.
Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Calveley VL, et al. Int J Radiat Biol. 2005 Dec;81(12):887-99. doi: 10.1080/09553000600568002. Int J Radiat Biol. 2005. PMID: 16524844 - Mechanisms and potential targets for prevention and treatment of normal tissue injury after radiation therapy.
Anscher MS, Vujaskovic Z. Anscher MS, et al. Semin Oncol. 2005 Apr;32(2 Suppl 3):S86-91. doi: 10.1053/j.seminoncol.2005.03.015. Semin Oncol. 2005. PMID: 16015541 Review. - Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury.
Slezak J, Kura B, Babal P, Barancik M, Ferko M, Frimmel K, Kalocayova B, Kukreja RC, Lazou A, Mezesova L, Okruhlicova L, Ravingerova T, Singal PK, Szeiffova Bacova B, Viczenczova C, Vrbjar N, Tribulova N. Slezak J, et al. Can J Physiol Pharmacol. 2017 Oct;95(10):1190-1203. doi: 10.1139/cjpp-2017-0121. Epub 2017 Jul 27. Can J Physiol Pharmacol. 2017. PMID: 28750189 Review.
Cited by
- Modeling radiation-induced lung injury: lessons learned from whole thorax irradiation.
Beach TA, Groves AM, Williams JP, Finkelstein JN. Beach TA, et al. Int J Radiat Biol. 2020 Jan;96(1):129-144. doi: 10.1080/09553002.2018.1532619. Epub 2018 Oct 25. Int J Radiat Biol. 2020. PMID: 30359147 Free PMC article. Review. - Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage.
Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Rebouças JS, Batinic-Haberle I, Vujaskovic Z. Gauter-Fleckenstein B, et al. Free Radic Biol Med. 2010 Apr 15;48(8):1034-43. doi: 10.1016/j.freeradbiomed.2010.01.020. Epub 2010 Jan 20. Free Radic Biol Med. 2010. PMID: 20096348 Free PMC article. - Dose Optimization Study of AEOL 10150 as a Mitigator of Radiation-Induced Lung Injury in CBA/J Mice.
Murigi FN, Mohindra P, Hung C, Salimi S, Goetz W, Pavlovic R, Jackson IL, Vujaskovic Z. Murigi FN, et al. Radiat Res. 2015 Oct;184(4):422-32. doi: 10.1667/RR14110.1. Epub 2015 Sep 28. Radiat Res. 2015. PMID: 26414508 Free PMC article. - Influence of endothelin 1 receptor inhibition on functional, structural and molecular changes in the rat heart after irradiation.
Boerma M, Wang J, Kulkarni A, Roberto KA, Qiu X, Kennedy RH, Hauer-Jensen M. Boerma M, et al. Radiat Res. 2008 Sep;170(3):275-83. doi: 10.1667/RR1093.1. Radiat Res. 2008. PMID: 18763854 Free PMC article. - Oxidative stress mediates radiation lung injury by inducing apoptosis.
Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Zhang Y, et al. Int J Radiat Oncol Biol Phys. 2012 Jun 1;83(2):740-8. doi: 10.1016/j.ijrobp.2011.08.005. Epub 2012 Jan 21. Int J Radiat Oncol Biol Phys. 2012. PMID: 22270165 Free PMC article.
References
- Abratt RP, Morgan GW. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer. 2002;35:103–109. - PubMed
- Roach M, 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13:2606–2612. - PubMed
- Moosavi H, McDonald S, Rubin P, et al. Early radiation dose-response in lung: an ultrastructural study. Int J Radiat Oncol Biol Phys. 1977;2:921–931. - PubMed
- Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 1980;6:345–347. - PubMed
- Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys. 1992;24:93–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA098452-03/CA/NCI NIH HHS/United States
- R01 CA098452-04/CA/NCI NIH HHS/United States
- P30 ES011961/ES/NIEHS NIH HHS/United States
- R01 CA098452-01A1/CA/NCI NIH HHS/United States
- ES 11961/ES/NIEHS NIH HHS/United States
- R01 CA098452/CA/NCI NIH HHS/United States
- R01 CA098452-02/CA/NCI NIH HHS/United States
- R01 CA 098452/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources