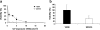Sonoporation is an efficient tool for intracellular fluorescent dextran delivery and one-step double-crossover mutant construction in Fusobacterium nucleatum - PubMed (original) (raw)
Comparative Study
. 2007 Jun;73(11):3677-83.
doi: 10.1128/AEM.00428-07. Epub 2007 Apr 20.
Affiliations
- PMID: 17449701
- PMCID: PMC1932673
- DOI: 10.1128/AEM.00428-07
Comparative Study
Sonoporation is an efficient tool for intracellular fluorescent dextran delivery and one-step double-crossover mutant construction in Fusobacterium nucleatum
Yiping W Han et al. Appl Environ Microbiol. 2007 Jun.
Abstract
Studies of microorganisms are often hindered by a lack of effective genetic tools. One such example is Fusobacterium nucleatum, a gram-negative anaerobe associated with various human infections, including those causing periodontal disease and preterm birth. The first double-crossover allelic-exchange mutant in F. nucleatum was recently constructed using sonoporation, a novel ultrasound-mediated intracellular delivery method, demonstrating potential for bacterial gene transfection. To better unveil its mechanism, the current study examines the factors affecting the outcome of sonoporation. Delivery of Texas Red-conjugated dextran into F. nucleatum by sonoporation was at least twice as efficient as that by electroporation, and sonoporation was nonbactericidal, unlike electroporation. The delivery efficiency was affected by the acoustic pressure amplitude, the duty cycle, and the quantity of microbubbles used to initiate cavitation but not by the pulse repetition frequency of ultrasound application. To examine the involvement of homologous recombination in sonoporation-mediated mutant construction, the highly conserved recA gene, which carried most of the consensus residues, including the P loop, was identified in F. nucleatum, and a double-crossover recA mutant of F. nucleatum 12230, US1610, was constructed by sonoporation. The mutant exhibited increased sensitivity to UV exposure compared with that of the wild type, indicating that the RecA function in F. nucleatum was conserved. Interestingly, US1610 was also sensitive to ultrasound treatment, suggesting the likely involvement of RecA in postsonoporation repair and survival. Since sonoporation has consistently generated one-step double-crossover mutants in F. nucleatum by use of intact suicide plasmids, this technology may be developed into an efficient tool for streamlining mutant construction in bacteria.
Figures
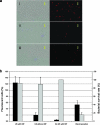
FIG. 1.
Delivery of Texas Red-conjugated dextran into F. nucleatum 12230 by sonoporation and electroporation. (a) i, fluorescent dextran uptake by F. nucleatum 12230 following sonoporation in the presence of Definity; ii, fluorescent dextran uptake by F. nucleatum 12230 without US treatment; and iii, fluorescent dextran uptake by F. nucleatum 12230 following electroporation. (b) Quantization of fluorescent dextran delivery into F. nucleatum 12230 following sonoporation in the presence of Definity, sonoporation in the absence of Definity, no US treatment, and electroporation (black bars, scales on the left). The bacterial survival rate following each treatment is shown with a gray bar (scales on the right). The results shown here are the averages for four individual experiments. The standard deviations are shown above the bars.
FIG. 2.
Effects of different sonoporation parameters, i.e., acoustic pressure amplitude (a), PRF and duty cycle (DC) (b), sonoporation duration (c), and quantity of Definity (d), on delivery of Texas Red-conjugated dextran into F. nucleatum 12230. The percentages of fluorescent F. nucleatum cells were calculated as follows: percent fluorescent cells = (number of fluorescent cells/total number of cells) × 100. Values are expressed as means and standard deviations (error bars) for three separate experiments performed in triplicate.
FIG. 3.
Nucleotide and deduced amino acid sequences of the recA gene of F. nucleatum 12230. The putative Shine-Dalgarno sequence (SD) is underlined. The consensus sequence of RecA is listed directly below the amino acid sequence. The 11 residues in F. nucleatum 12230 RecA different from those in the consensus sequence are underlined. The P-loop motif (GXXXXGKT) is boxed.
FIG. 4.
Construction of the _recA_-defective mutant US1610 of F. nucleatum 12230. (a) Schematic representation of the construction of the recA::ermF-ermAM mutant. The ermF-ermAM cassette was inserted at the HincII site in recA, conferring clindamycin resistance. (b) PCR analysis of F. nucleatum 12230 and US1610 chromosomes using primers _recA_F and _recA_2R. Lane 1, F. nucleatum 12230; lane 2, US1610. M, molecular size makers as indicated on the right. (c) Effects of different sonoporation conditions on the integrity of pYH1380. Lane 1, linearized pYH1380 following EcoRV digestion; lane 2, untreated intact pYH1380; lane 3, untreated intact pYH1380 in the presence of Definity; lanes 4, 5, 8, and 9, pYH1380 treated with sonoporation in the absence of Definity; lanes 6, 7, 10, and 11, pYH1380 treated with sonoporation in the presence of Definity. M, DNA molecular size markers. The sonoporation durations and the presence (+) or absence (−) of Definity are indicated below the gel. Each condition was tested in duplicate.
FIG. 5.
Susceptibilities of F. nucleatum 12230 and US1610 to UV (a) and sonoporation (b). Percent bacterial viability was expressed as the number of CFU following treatment over the total number of CFU prior to treatment. The sonoporation conditions used were 0.5 MPa, 1-Hz PRF, 90-s duration, 50% duty cycle, and 33% Definity (vol/vol). The standard deviations are indicated above and below the squares (a) or above the bars (b).
Similar articles
- Identification and characterization of a novel adhesin unique to oral fusobacteria.
Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. Han YW, et al. J Bacteriol. 2005 Aug;187(15):5330-40. doi: 10.1128/JB.187.15.5330-5340.2005. J Bacteriol. 2005. PMID: 16030227 Free PMC article. - Genetic Transformation of Fusobacterium nucleatum.
Yoshida A, Ikegami A. Yoshida A, et al. Methods Mol Biol. 2021;2210:43-50. doi: 10.1007/978-1-0716-0939-2_5. Methods Mol Biol. 2021. PMID: 32815126 - Native plasmids of Fusobacterium nucleatum: characterization and use in development of genetic systems.
Haake SK, Yoder SC, Attarian G, Podkaminer K. Haake SK, et al. J Bacteriol. 2000 Feb;182(4):1176-80. doi: 10.1128/JB.182.4.1176-1180.2000. J Bacteriol. 2000. PMID: 10648549 Free PMC article. - Advances in sonoporation strategies for cancer.
Zolochevska O, Figueiredo ML. Zolochevska O, et al. Front Biosci (Schol Ed). 2012 Jan 1;4(3):988-1006. doi: 10.2741/s313. Front Biosci (Schol Ed). 2012. PMID: 22202104 Review. - Fusobacterium nucleatum - Friend or foe?
Stokowa-Sołtys K, Wojtkowiak K, Jagiełło K. Stokowa-Sołtys K, et al. J Inorg Biochem. 2021 Nov;224:111586. doi: 10.1016/j.jinorgbio.2021.111586. Epub 2021 Aug 18. J Inorg Biochem. 2021. PMID: 34425476 Review.
Cited by
- Abiotic Gene Transfer: Rare or Rampant?
Kotnik T, Weaver JC. Kotnik T, et al. J Membr Biol. 2016 Oct;249(5):623-631. doi: 10.1007/s00232-016-9897-y. Epub 2016 Apr 11. J Membr Biol. 2016. PMID: 27067073 Free PMC article. Review. - Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery.
Kooiman K, Roovers S, Langeveld SAG, Kleven RT, Dewitte H, O'Reilly MA, Escoffre JM, Bouakaz A, Verweij MD, Hynynen K, Lentacker I, Stride E, Holland CK. Kooiman K, et al. Ultrasound Med Biol. 2020 Jun;46(6):1296-1325. doi: 10.1016/j.ultrasmedbio.2020.01.002. Epub 2020 Mar 10. Ultrasound Med Biol. 2020. PMID: 32165014 Free PMC article. Review. - Forward Genetic Dissection of Biofilm Development by Fusobacterium nucleatum: Novel Functions of Cell Division Proteins FtsX and EnvC.
Wu C, Al Mamun AAM, Luong TT, Hu B, Gu J, Lee JH, D'Amore M, Das A, Ton-That H. Wu C, et al. mBio. 2018 Apr 24;9(2):e00360-18. doi: 10.1128/mBio.00360-18. mBio. 2018. PMID: 29691334 Free PMC article. - Contrast agent-free sonoporation: The use of an ultrasonic standing wave microfluidic system for the delivery of pharmaceutical agents.
Carugo D, Ankrett DN, Glynne-Jones P, Capretto L, Boltryk RJ, Zhang X, Townsend PA, Hill M. Carugo D, et al. Biomicrofluidics. 2011 Dec;5(4):44108-4410815. doi: 10.1063/1.3660352. Epub 2011 Nov 15. Biomicrofluidics. 2011. PMID: 22662060 Free PMC article. - Signal peptide of FadA adhesin from Fusobacterium nucleatum plays a novel structural role by modulating the filament's length and width.
Témoin S, Wu KL, Wu V, Shoham M, Han YW. Témoin S, et al. FEBS Lett. 2012 Jan 2;586(1):1-6. doi: 10.1016/j.febslet.2011.10.047. Epub 2011 Nov 19. FEBS Lett. 2012. PMID: 22108653 Free PMC article.
References
- Deng, C. X., and F. L. Lizzi. 2002. A review of physical phenomena associated with ultrasonic contrast agents and illustrative clinical applications. Ultrasound Med. Biol. 28:277-286. - PubMed
- Deng, C. X., F. Sieling, H. Pan, and J. Cui. 2004. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 30:519-526. - PubMed
- Fenno, J. C., A. Shaikh, and P. Fives-Taylor. 1993. Characterization of allelic replacement in Streptococcus parasanguis: transformation and homologous recombination in a ‘nontransformable’ streptococcus. Gene 130:81-90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources