Type I interferon signaling is required for activation of the inflammasome during Francisella infection - PubMed (original) (raw)
Comparative Study
. 2007 May 14;204(5):987-94.
doi: 10.1084/jem.20062665. Epub 2007 Apr 23.
Affiliations
- PMID: 17452523
- PMCID: PMC2118578
- DOI: 10.1084/jem.20062665
Comparative Study
Type I interferon signaling is required for activation of the inflammasome during Francisella infection
Thomas Henry et al. J Exp Med. 2007.
Abstract
Francisella tularensis is a pathogenic bacterium whose virulence is linked to its ability to replicate within the host cell cytosol. Entry into the macrophage cytosol activates a host-protective multimolecular complex called the inflammasome to release the proinflammatory cytokines interleukin (IL)-1beta and -18 and trigger caspase-1-dependent cell death. In this study, we show that cytosolic F. tularensis subspecies novicida (F. novicida) induces a type I interferon (IFN) response that is essential for caspase-1 activation, inflammasome-mediated cell death, and release of IL-1beta and -18. Extensive type I IFN-dependent cell death resulting in macrophage depletion occurs in vivo during F. novicida infection. Type I IFN is also necessary for inflammasome activation in response to cytosolic Listeria monocytogenes but not vacuole-localized Salmonella enterica serovar Typhimurium or extracellular adenosine triphosphate. These results show the specific connection between type I IFN signaling and inflammasome activation, which are two sequential events triggered by the recognition of cytosolic bacteria. To our knowledge, this is the first example of the positive regulation of inflammasome activation. This connection underscores the importance of the cytosolic recognition of pathogens and highlights how multiple innate immunity pathways interact before commitment to critical host responses.
Figures
Figure 1.
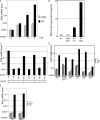
WT cytosolic F. novicida induces a type I IFN transcriptional response in BMMs, whereas the vacuole-restricted mglA mutant does not. At 8 h postinfection (PI), 68 genes were statistically differentially regulated between mglA and WT _F. novicida_–infected BMMs. The arrays corresponding to the biological replicates and the 21 genes with the higher changes between mglA and WT infected macrophages are shown.
Figure 2.
F. novicida in the host cytosol induces IFN-β secretion in a TLR-independent IRF-3–dependent manner. IFN-β mRNA levels were determined by quantitative RT-PCR in uninfected BMMs (Un) or at various times PI (A) and 7 (C), 9 (D), or 8 (E) h PI with either mglA or WT F. novicida. IFN-β levels were determined by ELISA in the supernatant of BMMs infected at the indicated MOI for 9 h (ND, nondetectable; B). Various vacuole-restricted mutants do not induce IFN-β mRNA, whereas their complemented counterparts (c.) do. Cytosolic localization is shown (C). BMMs from WT or from IRF-3−/−, ASC−/−, MyD88/TRIFDKO, Ipaf−/−, RIP2−/− (D), MAVS−/− mice, or WT littermates (E) were analyzed for their IFN-β mRNA levels. Error bars represent SEM.
Figure 3.
Type I IFN induction and signaling is required for _F. novicida_–mediated but not for _S. typhimurium_–mediated cell death. Cell death of WT, IFNR−/−, ASC−/−, and caspase-1 (casp1)−/− BMMs was assayed by lactate dehydrogenase (LDH) release. BMMs either unactivated (B) or preactivated with heat-killed F. novicida (A, C, and D [left]) or pretreated with recombinant IFN-β (D, right) were infected for 8 (A), 12.5 (B), 3 (C), or 6 h (D) with F. novicida (A, B, and D) or S. typhimurium (C) strains at the indicated MOI. In agreement with previous data (30), cell death required the S. typhimurium gene sipB. Error bars represent SEM.
Figure 4.
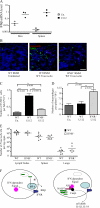
Type I IFN signaling is necessary for activation of the inflammasome during F. novicida and L. monocytogenes infections but not upon ATP treatment. (A) WT, IFNR−/−, and ASC−/− BMMs uninfected (Un) or infected with mglA (m) or WT F. novicida (W) were lysed at 9 h PI. Caspase-1 processing was visualized by detection of the p20 subunit only in WT macrophages infected with WT F. novicida. (B and C) IL-1β (B) and -18 (C) were quantified by ELISA in the supernatant of preactivated BMMs. Similar levels were detected in WT and IFNR−/− BMMs treated for 3 h with 5 mM ATP (left), whereas high levels were only detected in WT macrophages upon infection with F. novicida for 5 h (right). (D) Cell death (left) was strongly reduced in IFNR−/− compared with WT BMMs at 6 h PI with L. monocytogenes. Similarly, IL-1β (middle) and -18 (right) levels were lower in activated BMMs infected for 2.5 h with L. monocytogenes at the indicated MOI.
Figure 5.
IFN-β is up-regulated in vivo upon infection with F. novicida, leading to IFNR-dependent cell death and depletion of BMMs in the spleen at 48 h PI. Each symbol represents the value of one individual mouse. Horizontal bars correspond to the geometric mean. (A) IFN-β mRNA levels in the skin and spleen of WT uninfected (open symbols) or infected (closed symbols) mice were determined by quantitative RT-PCR. (B) TUNEL staining (green) performed on a spleen section of uninfected (left), infected (middle), WT, or infected IFNR−/− mice (right) nuclei (blue), and F. novicida (red) stainings are shown. Representative images are shown. Bar, 100 μm. (C–E) Quantification of the number of TUNEL-positive cells (C), splenic macrophages (D), and F. novicida CFU in various organs from WT (closed symbols) and IFNR−/− (open symbols) mice (E) are presented. (F) Model for the inflammasome activation in response to F. novicida (see Conclusion and model: cytosolic sensing of F. novicida … for details). Error bars represent SEM.
Similar articles
- Francisella tularensis: activation of the inflammasome.
Weiss DS, Henry T, Monack DM. Weiss DS, et al. Ann N Y Acad Sci. 2007 Jun;1105:219-37. doi: 10.1196/annals.1409.005. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395724 Review. - ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida.
Pierini R, Perret M, Djebali S, Juruj C, Michallet MC, Förster I, Marvel J, Walzer T, Henry T. Pierini R, et al. J Immunol. 2013 Oct 1;191(7):3847-57. doi: 10.4049/jimmunol.1203326. Epub 2013 Aug 23. J Immunol. 2013. PMID: 23975862 - cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection.
Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. Storek KM, et al. J Immunol. 2015 Apr 1;194(7):3236-45. doi: 10.4049/jimmunol.1402764. Epub 2015 Feb 20. J Immunol. 2015. PMID: 25710914 Free PMC article. - IFN-γ extends the immune functions of Guanylate Binding Proteins to inflammasome-independent antibacterial activities during Francisella novicida infection.
Wallet P, Benaoudia S, Mosnier A, Lagrange B, Martin A, Lindgren H, Golovliov I, Michal F, Basso P, Djebali S, Provost A, Allatif O, Meunier E, Broz P, Yamamoto M, Py BF, Faudry E, Sjöstedt A, Henry T. Wallet P, et al. PLoS Pathog. 2017 Oct 2;13(10):e1006630. doi: 10.1371/journal.ppat.1006630. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 28968459 Free PMC article. - Francisella Inflammasomes: Integrated Responses to a Cytosolic Stealth Bacterium.
Wallet P, Lagrange B, Henry T. Wallet P, et al. Curr Top Microbiol Immunol. 2016;397:229-56. doi: 10.1007/978-3-319-41171-2_12. Curr Top Microbiol Immunol. 2016. PMID: 27460813 Review.
Cited by
- Mouse guanylate-binding proteins of the chromosome 3 cluster do not mediate antiviral activity in vitro or in mouse models of infection.
Tessema MB, Feng S, Enosi Tuipulotu D, Farrukee R, Ngo C, Gago da Graça C, Yamomoto M, Utzschneider DT, Brooks AG, Londrigan SL, Man SM, Reading PC. Tessema MB, et al. Commun Biol. 2024 Aug 25;7(1):1050. doi: 10.1038/s42003-024-06748-8. Commun Biol. 2024. PMID: 39183326 Free PMC article. - Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses.
Stewart MK, Cookson BT. Stewart MK, et al. Nat Rev Microbiol. 2016 Jun;14(6):346-59. doi: 10.1038/nrmicro.2016.50. Epub 2016 May 13. Nat Rev Microbiol. 2016. PMID: 27174147 Review. - AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages.
Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. Pierini R, et al. Cell Death Differ. 2012 Oct;19(10):1709-21. doi: 10.1038/cdd.2012.51. Epub 2012 May 4. Cell Death Differ. 2012. PMID: 22555457 Free PMC article. - Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella.
Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Broz P, et al. J Exp Med. 2010 Aug 2;207(8):1745-55. doi: 10.1084/jem.20100257. Epub 2010 Jul 5. J Exp Med. 2010. PMID: 20603313 Free PMC article. - Targeting innate sensing in the tumor microenvironment to improve immunotherapy.
Liu Z, Han C, Fu YX. Liu Z, et al. Cell Mol Immunol. 2020 Jan;17(1):13-26. doi: 10.1038/s41423-019-0341-y. Epub 2019 Dec 16. Cell Mol Immunol. 2020. PMID: 31844141 Free PMC article. Review.
References
- Janeway, C.A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54:1–13. - PubMed
- Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. - PubMed
- Decker, T., M. Muller, and S. Stockinger. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5:675–687. - PubMed
- Girardin, S.E., I.G. Boneca, L.A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M.K. Taha, A. Labigne, U. Zahringer, et al. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 300:1584–1587. - PubMed
- Girardin, S.E., I.G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D.J. Philpott, and P.J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869–8872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases