Murine cerebral malaria development is independent of toll-like receptor signaling - PubMed (original) (raw)
Murine cerebral malaria development is independent of toll-like receptor signaling
Dieudonnée Togbe et al. Am J Pathol. 2007 May.
Abstract
Malaria pigment hemozoin was reported to activate the innate immunity by Toll-like receptor (TLR)-9 engagement. However, the role of TLR activation for the development of cerebral malaria (CM), a lethal complication of malaria infection in humans, is unknown. Using Plasmodium berghei ANKA (PbA) infection in mice as a model of CM, we report here that TLR9-deficient mice are not protected from CM. To exclude the role of other members of the TLR family in PbA recognition, we infected mice deficient for single TLR1, -2, -3, -4, -6, -7, or -9 and their adapter proteins MyD88, TIRAP, and TRIF. In contrast to lymphotoxin alpha-deficient mice, which are resistant to CM, all TLR-deficient mice were as sensitive to fatal CM development as wild-type control mice and developed typical microvascular damage with vascular leak and hemorrhage in the brain and lung, together with comparable parasitemia, thrombocytopenia, neutrophilia, and lymphopenia. In conclusion, the present data do not exclude the possibility that malarial molecular motifs may activate the innate immune system. However, TLR-dependent activation of innate immunity is unlikely to contribute significantly to the proinflammatory response to PbA infection and the development of fatal CM.
Figures
Figure 1
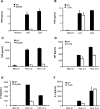
Cytokine production by macrophages derived from MyD88- and TLR9-deficient mice. Bone marrow-derived macrophages from WT, MyD88-deficient (MyD88), or TLR9-deficient (TLR9) mice were stimulated with LPS (100 ng/ml), CpG (0.125 μmol/L), and noninfected (RBC NI) or parasitized red blood cells (PbA at 3 or 10 × 105 cells per ml). After 20 hours, the culture supernatants were analyzed for concentrations of TNF (A–D) and IL-6 (E, F). Results are from one representative experiment (n = 2 mice per genotype) of three independent experiments; the differences in response to LPS, CpG, and PbA between WT and MyD88-deficient mice were all statistically significant whereas statistically significant differences between WT controls and TLR9-deficient mice were seen merely for CpG and PbA 10× stimulated TNF (P < 0.05).
Figure 2
Survival from PbA-infected TLR-deficient mice. WT mice or those deficient for TLR9 or LT-α (A); TLR1 or TLR6 (B); TLR2 alone or plus TLR4 (C); TLR4 or CD14 (D); TLR3 (E); or MyD88, TIRAP, or TRIF (F) were infected with 106 PbA_-_parasitized red blood cells and the survival monitored daily. Results are given as Kaplan-Meyer curves of n = 7 mice per group pooled from two independent experiments. There was no statistically significant difference between the WT controls and the genetically deficient mice, except for LT-α-deficient mice (P < 0.001).
Figure 3
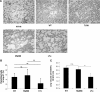
Cerebral microvascular leak and lesion in mice developing CM. Vascular leak as assessed macroscopically (A) by the blue discoloration of the brain of control WT, MyD88-deficient, and LT-α-deficient mice injected intravenously with 1% Evans blue on day 7 with severe CM or quantified after extravasation in formamide (C). Microvascular damage with mononuclear cell adhesion and perivascular hemorrhage (B) and ICAM-1 expression by immunohistochemistry on microvessels (D) in WT, MyD88-deficient, and LT-α-deficient mice. The data represent the mean ± SD of n = 5 mice per group pooled from two independent experiments (**P < 0.01); n.s., not significant. NI, noninfected.
Figure 4
Acute pulmonary inflammation, hemorrhage, and parasite sequestration in mice developing CM. A: Pulmonary inflammation, edema, and hemorrhage in the alveolar space was evaluated in H&E-stained microscopic sections of WT mice and those deficient for TLR9, MyD88, or LT-α 7 days after infection with 106 PbA-parasitized red blood cells. B: Parasite sequestration in the lung was analyzed by flow cytometry of WT, MyD88-deficient, and LT-α-deficient mice 7 days after infection with 106 enhanced green fluorescent protein (EGFP)_-_PbA parasitized red blood cells. C: Pulmonary vascular leak was quantified by lung Evans blue extravasation in formamide on day 7 with severe CM. The data represent the mean ± SD of n = 5 mice per group, from one of two independent experiments (**P < 0.01; n.s., not significant). NI, noninfected mice. Original magnifications, ×40.
Figure 5
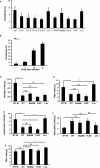
Parasitemia and hematological alterations are TLR-independent. The percentage of parasitized erythrocytes in the peripheral blood was determined by analyzing GFP fluorescent erythrocytes using flow cytometry (A) on day 7 for all TLR pathway-deficient mice and on days 7, 11, 18, and 22 after infection for resistant LT-α-deficient mice (B). Total platelet, leukocyte, lymphocyte, neutrophil, and erythrocyte counts were determined in the peripheral blood of control WT and MyD88-, TLR2-, and LT-α-deficient mice (C–G). The data represent the mean ± SD of n = 5 mice per group from two independent experiments (*P < 0.05; **P < 0.01). NI, noninfected mice. n.s., not significant.
Figure 6
Hepatic parasite sequestration and hemoglobin-derived pigment deposition is TLR-independent. A: Microscopic analysis of liver sections from control WT and MyD88- and TLR9-deficient mice at day 7 of infection, H&E staining. B: Sequestration of GFP-labeled parasites in the liver by flow cytometry analysis of liver homogenates. The data represent the mean ± SD of n = 5 mice per group from two independent experiments (NI, noninfected; n.s., not significant). Original magnifications, ×40.
Similar articles
- Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.
Malasala S, Azimian F, Chen YH, Twiss JL, Boykin C, Akhtar SN, Lu Q. Malasala S, et al. bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. PMID: 38260445 Free PMC article. Updated. Preprint. - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article. - Pharmacological treatments in panic disorder in adults: a network meta-analysis.
Guaiana G, Meader N, Barbui C, Davies SJ, Furukawa TA, Imai H, Dias S, Caldwell DM, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A, Dawson S, Robertson L. Guaiana G, et al. Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
- Systemic release of high mobility group box 1 (HMGB1) protein is associated with severe and fatal Plasmodium falciparum malaria.
Higgins SJ, Xing K, Kim H, Kain DC, Wang F, Dhabangi A, Musoke C, Cserti-Gazdewich CM, Tracey KJ, Kain KC, Liles WC. Higgins SJ, et al. Malar J. 2013 Mar 19;12:105. doi: 10.1186/1475-2875-12-105. Malar J. 2013. PMID: 23506269 Free PMC article. - Hemozoin activates the innate immune system and reduces Plasmodium berghei infection in Anopheles gambiae.
Simões ML, Gonçalves L, Silveira H. Simões ML, et al. Parasit Vectors. 2015 Jan 8;8:12. doi: 10.1186/s13071-014-0619-y. Parasit Vectors. 2015. PMID: 25573379 Free PMC article. - Pathogenic roles of CD14, galectin-3, and OX40 during experimental cerebral malaria in mice.
Oakley MS, Majam V, Mahajan B, Gerald N, Anantharaman V, Ward JM, Faucette LJ, McCutchan TF, Zheng H, Terabe M, Berzofsky JA, Aravind L, Kumar S. Oakley MS, et al. PLoS One. 2009 Aug 27;4(8):e6793. doi: 10.1371/journal.pone.0006793. PLoS One. 2009. PMID: 19710907 Free PMC article. - Divergent roles of IRAK4-mediated innate immune responses in two experimental models of severe malaria.
Finney CA, Lu Z, Hawkes M, Yeh WC, Liles WC, Kain KC. Finney CA, et al. Am J Trop Med Hyg. 2010 Jul;83(1):69-74. doi: 10.4269/ajtmh.2010.09-0753. Am J Trop Med Hyg. 2010. PMID: 20595480 Free PMC article. - Dysregulation of coagulation in cerebral malaria.
Moxon CA, Heyderman RS, Wassmer SC. Moxon CA, et al. Mol Biochem Parasitol. 2009 Aug;166(2):99-108. doi: 10.1016/j.molbiopara.2009.03.006. Epub 2009 Mar 26. Mol Biochem Parasitol. 2009. PMID: 19450727 Free PMC article. Review.
References
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. - PubMed
- Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. - PubMed
- Rudin W, Eugster HP, Bordmann G, Bonato J, Muller M, Yamage M, Ryffel B. Resistance to cerebral malaria in tumor necrosis factor-alpha/beta-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol. 1997;150:257–266. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases