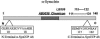Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation - PubMed (original) (raw)
Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation
Brian M Dufty et al. Am J Pathol. 2007 May.
Abstract
Parkinson's disease (PD) and dementia with Lewy bodies (DLB) are both characterized pathologically by the presence of neuronal inclusions termed Lewy bodies (LBs). A common feature found in LBs are aggregates of alpha-synuclein (alpha-Syn), and although it is now recognized that alpha-Syn is the major building block for these toxic filaments, the mechanism of how this occurs remains unknown. In the present study, we demonstrate that proteolytic processing of alpha-Syn by the protease calpain I leads to the formation of aggregated high-molecular weight species and adoption of a beta-sheet structure. To determine whether calpain-cleavage of alpha-Syn occurs in PD and DLB, we designed site-directed calpain-cleavage antibodies to alpha-Syn and tested their utility in several animal model systems. Detection of calpain-cleaved alpha-Syn was evident in mouse models of cerebral ischemia and PD and in a Drosophila model of PD. In the human PD and DLB brain, calpain-cleaved alpha-Syn antibodies immunolabeled LBs and neurites in the substantia nigra. Moreover, calpain-cleaved alpha-Syn fragments identified within LBs colocalized with activated calpain in neurons of the PD and DLB brains. These findings suggest that calpain I may participate in the disease-linked aggregation of alpha-Syn in various alpha-synucleinopathies.
Figures
Figure 1
Peptide sequences used to generate site-directed calpain-cleavage Abs and locations of the epitopes for the full-length anti-α-Syn Abs used in the present study. Sequences between amino acids 10 and 18 or 117 and 122 were used to synthesize peptides and generate polyclonal Abs as described in Materials and Methods. The N-terminal site was chosen based on sequence analysis after digestion of recombinant α-Syn with calpain I, whereas the C-terminal site was chosen based on a previous report demonstrating the cleavage of α-Syn between amino acids 122 and 123. Reported epitopes for the two full-length anti-α-Syn Abs (LB509 and AB5038) used in this study are also shown.
Figure 2
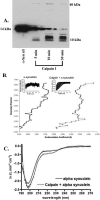
Calpain cleavage of α-synuclein results in aggregation and adoption of a β-sheet configuration. A: After incubation of α-Syn with calpain I for various periods of time in a cell-free assay, samples were subjected to gel electrophoresis, transferred to nitrocellulose filters, and probed with an antibody to FL α-Syn (AB5038). Over time, the appearance of low- and high-molecular weight bands were evident in addition to the disappearance of FL α-Syn (14 kd). B: Analytical ultracentrifugation analysis after incubation of α-Syn alone (line on the left) or after digestion with calpain I (line on the right) for 30 minutes indicated the presence of high-molecular weight species. Insets represent the 30 raw data scans used for data analysis. C: CD spectra after incubation of α-Syn with calpain I indicated a change in structure from random coil to a β-sheet configuration. CD spectra of α-Syn alone (top line) and mixture of α-Syn incubated with calpain I with the calpain spectrum subtracted (bottom line).
Figure 3
Calpain cleavage of α-Syn results in aggregation and formation of high-molecular weight species. A: After incubation of α-Syn with calpain I for various periods of time in a cell-free assay, samples were subjected to gel electrophoresis, transferred to polyvinylidene difluoride filters, and stained with Coomassie blue. Over time, the appearance of low- and high-molecular weight bands were evident in addition to the disappearance of full-length α-Syn (14 kd). B: Western blot analysis reveals the presence of high-molecular weight bands after digestion of α-Syn with calpain I in a cell-free system. α-Syn was incubated with calpain I for the indicated times, and samples representing the soluble fractions were immunoblotted using the α-SynCCP Ab. The arrowhead indicates the immunoreactivity of the α-SynCCP Ab with a 10-kd fragment of α-Syn. C: Western blot analysis using the α-SynCCP Ab demonstrated the presence of calpain-cleaved aggregates of α-Syn after treatment of SY5Y neuroblastoma cells with the calcium ionophore A23187 (C, lanes marked 2 and 4 hours), which were prevented after pretreatment of cells with a calpain I inhibitor (D, far right lane). E: Detection of numerous high-molecular weight calpain-cleavage aggregates of α-Syn in the DLB brain by Western blot analysis. Human brain lysates from age-matched controls or DLB cases were probed with the α-SynCCP antibody. Arrowheads above 10 kd indicate the presence of bands between 30 and 80 kd.
Figure 4
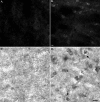
Detection of calpain-cleaved α-Syn in an animal model of cerebral ischemia. Mice were subjected to 1 hour of MCAO, followed by reperfusion for 24 hours. Sections at 50 μm were subjected to either IF or IH analysis using the α-SynCCP Ab (1:100) to detect calpain-cleaved α-Syn. Staining of shrunken, damaged neurons in the cortex of MCAO mice was evident only in ischemic infarct areas (B, fluorescence, and D, bright-field DAB labeling) and was not evident on the contralateral side of the brain (A and C). Scale bars = 10 μm.
Figure 5
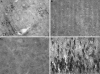
Detection of calpain-cleaved α-Syn in a transgenic mouse model of PD. Fixed-tissue brain sections from age-matched non-Tg controls in the hippocamus (A) or cortex (B) immunolabeled with the α-SynCCP Ab (1:100). C and D: Cortical staining from 4-month-old asymptomatic PD Tg mice (C) or 12-month-old PD Tg mice (D) was immunolabeled with the α-SynCCP Ab (1:100). Scale bars = 10 μm.
Figure 6
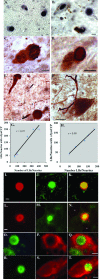
Detection of calpain-cleaved species of α-Syn in PD and DLB. A and B: Representative immunohistochemical single-label staining in the substantia nigra of a DLB (A) and PD (B) case after application of the α-SynCCP Ab demonstrates the presence of calpain-cleaved α-Syn within Lewy bodies. C and D: Double-label immunohistochemical analysis with α-SynCCP Ab (black) together with a marker for Lewy bodies (LB509, orange) reveals colocalization in Lewy bodies from a representative DLB (C) or PD (D) case. Colocalization was also observed in Lewy neurites in DLB (E, arrows) or PD (F) cases. G and H: Graphical representations depicting the relationship between calpain-cleaved α-Syn and the number of LBs/neurites in five different DLB (left) or PD (right) cases. Data indicate a high degree of association between the total number of LBs/neurites and the presence of calpain-cleaved α-Syn. I–N: Double-label immunofluorescence images for α-SynCCP (green) and an antibody to full-length α-Syn (red) confirmed the colocalization of calpain-cleaved α-Syn within Lewy bodies in a representative DLB (I–K) or PD (L–N) case. Yellow/orange coloring in K and N indicate areas where markers are overlapping. O–T: Double-label immunofluorescence images for α-SynCCP (green) and calpain I (red) demonstrating the presence of active fragments of the enzyme surrounding Lewy bodies containing calpain-cleaved α-Syn. O–Q and R–T: Representative staining of a DLB or PD case, respectively. Yellow/orange coloring in Q and T indicate areas where markers are overlapping. Asterisks in N denote regions containing neuromelanin. All scale bars = 10 μm.
Figure 7
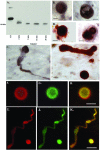
Evidence for C-terminal truncation of α-Syn in the DLB brain. A and B: Characterization of the C-terminal α-SynCCP Ab by Western blot analysis. Recombinant human α-Syn (20 μg) was incubated for various periods of time with calpain I (2 U), separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose, and probed with an antibody directed toward a predicted C-terminal calpain-cleavage site (PVDPDN) of α-Syn (A). The antibody immunolabeled the predicted 10-kd fragment of α-Syn. B: Representative immunohistochemical single-label staining of LBs in the substantia nigra of two DLB cases after application of the C-terminal α-SynCCP Ab. C: Identical to B, showing labeling with a single Lewy neurite. D and E: Double-label immunohistochemical analysis in a representative DLB case showing localization of the C-terminal α-SynCCP Ab within LBs and neurites (nickel DAB), which colocalized with a marker for LBs (Nova Red). F–K: Immunofluorescence with the C-terminal α-SynCCP Ab shown in green (G and J), FL α-Syn Ab (LB509) in red (F and I), and the overlap image (H and K). All scale bars = 10 μm.
Figure 8
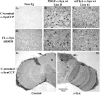
Comparison of C-terminal calpain-cleaved α-Syn immunoreactivity from several different animal models of PD. A–F: Panels are from vibratome sections from the brains of mice immunostained with either the C-terminal α-SynCCP Ab (A–C) or a commercially available FL α-Syn Ab from Chemicon AB5038 (D–F). After application of the C-terminal α-SynCCP Ab, immunoreactivity within neurons of the neocortex was observed in two different transgenic mice expressing wild-type α-Syn under a PDGF promoter (B) or in a line expressing α-Syn under Thy-1 promoter (C). E and F: A similar staining profile was observed using the FL α-Syn Ab. A and D: No staining with either antibody was observed in non-Tg control mice. G and H: Detection of the C-terminal calpain-cleavage fragment of α-Syn in a Drosophila model of PD. Paraffin-embedded frontal brain sections at the level of the mushroom bodies from 20-day-old flies were immunostained with the C-terminal α-SynCCP Ab (DAB staining) and counterstained with hematoxylin. G: The control genotype (elav-GAL/+). H: α-Syn expressed in a pan-neural pattern under the control of the elav-GAL driver. Arrows, the peripheral cortex; asterisks, the inner neuropil.
Figure 9
Detection of the C-terminal calpain-cleavage fragment of α-Syn by Western blot analysis. Cytosolic and membrane fractions were prepared from nonTg mice (lanes 1 and 2), Tg mice overexpressing wild-type or mutant A53T α-Syn under the PDGF promoter (lanes 3 and 4), or from two representative DLB cases (lanes 5 and 6). Blots were probed either with the C-terminal α-SynCCP Ab (A) or with mAb LB509 (B). The C-terminal fragment of α-Syn was detected by α-SynCCP Ab in soluble fractions but not by LB509 (band running just under full-length, 14 kd). C: IP experiments with the C-terminal α-SynCCP Ab incubated with control α-Syn (lane 1) or digested with calpain I (lane 2) under nondenaturing or denaturing conditions. Data suggest the C-terminal α-SynCCP Ab is a conformational-specific Ab whose epitope is lost after denaturation of α-Syn.
Similar articles
- FKBP12-immunopositive inclusions in patients with α-synucleinopathies.
Honjo Y, Ayaki T, Horibe T, Ito H, Takahashi R, Kawakami K. Honjo Y, et al. Brain Res. 2018 Feb 1;1680:39-45. doi: 10.1016/j.brainres.2017.12.012. Epub 2017 Dec 12. Brain Res. 2018. PMID: 29246765 - Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and Dementia with Lewy bodies.
Ferrer I, Blanco R, Carmona M, Puig B, Barrachina M, Gómez C, Ambrosio S. Ferrer I, et al. J Neural Transm (Vienna). 2001;108(12):1383-96. doi: 10.1007/s007020100015. J Neural Transm (Vienna). 2001. PMID: 11810403 - Caspase-cleaved transactivation response DNA-binding protein 43 in Parkinson's disease and dementia with Lewy bodies.
Kokoulina P, Rohn TT. Kokoulina P, et al. Neurodegener Dis. 2010;7(4):243-50. doi: 10.1159/000287952. Epub 2010 May 5. Neurodegener Dis. 2010. PMID: 20551689 Free PMC article. - Formation and development of Lewy pathology: a critical update.
Jellinger KA. Jellinger KA. J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review. - α-synuclein aggregation and its modulation.
Ghosh D, Mehra S, Sahay S, Singh PK, Maji SK. Ghosh D, et al. Int J Biol Macromol. 2017 Jul;100:37-54. doi: 10.1016/j.ijbiomac.2016.10.021. Epub 2016 Oct 11. Int J Biol Macromol. 2017. PMID: 27737778 Review.
Cited by
- Design, synthesis, and optimization of novel epoxide incorporating peptidomimetics as selective calpain inhibitors.
Schiefer IT, Tapadar S, Litosh V, Siklos M, Scism R, Wijewickrama GT, Chandrasena EP, Sinha V, Tavassoli E, Brunsteiner M, Fa' M, Arancio O, Petukhov P, Thatcher GR. Schiefer IT, et al. J Med Chem. 2013 Aug 8;56(15):6054-68. doi: 10.1021/jm4006719. Epub 2013 Jul 22. J Med Chem. 2013. PMID: 23834438 Free PMC article. - Time course and progression of wild type α-synuclein accumulation in a transgenic mouse model.
Amschl D, Neddens J, Havas D, Flunkert S, Rabl R, Römer H, Rockenstein E, Masliah E, Windisch M, Hutter-Paier B. Amschl D, et al. BMC Neurosci. 2013 Jan 9;14:6. doi: 10.1186/1471-2202-14-6. BMC Neurosci. 2013. PMID: 23302418 Free PMC article. - Calcium, Bioenergetics, and Parkinson's Disease.
Zampese E, Surmeier DJ. Zampese E, et al. Cells. 2020 Sep 8;9(9):2045. doi: 10.3390/cells9092045. Cells. 2020. PMID: 32911641 Free PMC article. Review. - Inhibition of calpain prevents manganese-induced cell injury and alpha-synuclein oligomerization in organotypic brain slice cultures.
Xu B, Liu W, Deng Y, Yang TY, Feng S, Xu ZF. Xu B, et al. PLoS One. 2015 Mar 10;10(3):e0119205. doi: 10.1371/journal.pone.0119205. eCollection 2015. PLoS One. 2015. PMID: 25756858 Free PMC article. - Immunotherapies in Huntington's disease and α-Synucleinopathies.
Fatoba O, Ohtake Y, Itokazu T, Yamashita T. Fatoba O, et al. Front Immunol. 2020 Feb 25;11:337. doi: 10.3389/fimmu.2020.00337. eCollection 2020. Front Immunol. 2020. PMID: 32161599 Free PMC article. Review.
References
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. - PubMed
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. - PubMed
- Gibb WR. Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathol Appl Neurobiol. 1986;12:223–234. - PubMed
- Barber R, Panikkar A, McKeith IG. Dementia with Lewy bodies: diagnosis and management. Int J Geriatr Psychiatry. 2001;16(Suppl 1):S12–S18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous