Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors - PubMed (original) (raw)
Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors
Norman L Lehman et al. Am J Pathol. 2007 May.
Abstract
The fidelity of cell division is dependent on the accumulation and ordered destruction of critical protein regulators. By triggering the appropriately timed, ubiquitin-dependent proteolysis of the mitotic regulatory proteins securin, cyclin B, aurora A kinase, and polo-like kinase 1, the anaphase promoting complex/cyclosome (APC/C) ubiquitin ligase plays an essential role in maintaining genomic stability. Misexpression of these APC/C substrates, individually, has been implicated in genomic instability and cancer. However, no comprehensive survey of the extent of their misregulation in tumors has been performed. Here, we analyzed more than 1600 benign and malignant tumors by immunohistochemical staining of tissue microarrays and found frequent overexpression of securin, polo-like kinase 1, aurora A, and Skp2 in malignant tumors. Positive and negative APC/C regulators, Cdh1 and Emi1, respectively, were also more strongly expressed in malignant versus benign tumors. Clustering and statistical analysis supports the finding that malignant tumors generally show broad misregulation of mitotic APC/C substrates not seen in benign tumors, suggesting that a "mitotic profile" in tumors may result from misregulation of the APC/C destruction pathway. This profile of misregulated mitotic APC/C substrates and regulators in malignant tumors suggests that analysis of this pathway may be diagnostically useful and represent a potentially important therapeutic target.
Figures
Figure 1
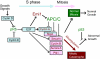
Model for pRb- and APC/C-dependent control of S phase and early mitosis. G1 proliferation control genes upstream of Emi1 (shown in blue) regulate the E2F-dependent expression of Emi1 and certain APC/C substrates (cyclin A, Plk1, and securin). Accumulation of Emi1 stabilizes APC/C substrates vital for progression through S phase (blue) and mitosis (pink). When overexpressed Emi1, or the mitotic control APC/C substrates Skp2, Plk1, securin, or aurora A can induce mitotic catastrophe. In the absence of p53, these genomically unstable cells survive and lead to tumor formation.
Figure 2
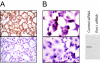
Validation of anti-Emi1 immunohistochemical staining. A: An Emi1-immunopositive ovarian clear cell carcinoma was stained with anti-Emi1 antibody showing characteristic cytoplasmic Emi1 immunoreactivity (top) or antibody preincubated with recombinant antigen eliminating Emi1-specific immunostaining (bottom). B: HCT116 cells transfected with control siRNAs specific for green fluorescent protein (top) or siRNAs specific for Emi1 (bottom) were fixed and immunostained for Emi1. Cells transfected with Emi1 siRNA express considerably less Emi1 as indicated by the decrease in red AEC chromagen labeling of the cells and elimination of the Emi1 band by Western blot (right).
Figure 3
Analysis of Emi1 protein expression in human tumors. Emi1 is highly expressed in retinoblastoma (eye whole mount and retinoblastoma rosette) and other major tumor types (TMA cores). The viable peripheral tumor tissue within the eye whole mount is Emi1 immunopositive, whereas the necrotic center is nonreactive. Normal lymph nodes and breast tissue exhibit less intense Emi1 staining than lymphomas and breast ductal cancer, respectively. Benign fibrocystic breast tissue and endometrial stroma show very little Emi1 staining in the ductal carcinoma in situ and endometrial cancer cores, respectively. Positive immunoreactivity is indicated by the brown diaminobenzidine chromagen.
Figure 4
Summary of TMA analysis of Emi1 and APC/C substrate protein expression in human tumors. TMAs representing different classes of tumors, grouped according to developmental tissue origin, were analyzed by immunohistochemical staining. Numbers in boxes indicate the percentage of positive tumors (specific numbers of immunopositive and total tumors surveyed are summarized in Supplemental Table 2, see http://ajp.amjpathol.org). Green indicates a low percentage of immunopositive tumors (<33% of cases); dark red, an intermediate percentage (33 to 66%); and bright red, a high percentage of Emi1-positive tumors (>66%). Benign tumors are highlighted by the blue boxes and less aggressive malignant tumors by yellow boxes. World Health Organization grades are indicated for astrocytomas and follicular lymphomas. Histopathological grades are also listed for colon adenocarcinomas. Adenoca, adenocarcinoma; Ca, carcinoma; CIS, carcinoma in situ; DFSP, dermatofibrosarcoma protuberans; esoph., esophageal; GI, gastrointestinal; HN, head and neck; MPNST, malignant peripheral nerve sheath tumor; PNET, primitive neuroectodermal tumor.
Figure 5
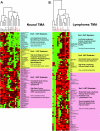
Hierarchical clustering analysis of Emi1, proliferation control, and mitotic control proteins in human tumors. For each tumor type, immunopositivity for Emi1, proliferation control (cyclins D1, D2, E, and A; β-catenin; p27Kip1; Bcl2; and Ki67), and mitotic control/APC/C (aurora A, Plk1, securin, Cdh1, and Skp2) substrates were identified. Dendrograms for neural tumor (A) and lymphoma (B) TMAs are shown. Green rectangles indicate a tumor showing no immunopositivity, dark red indicates moderate positivity (3 to 29% of tumor cells positive), and bright red indicates high positivity (>30% tumor cells positive). Gray rectangles indicate unscorable TMA cores. The dendograms on the top horizontal axes show clusters of proliferation control proteins and APC/C substrate proteins. Oncogenic APC/C substrates securin, aurora A, Plk1, and Skp2 (red) form a cluster with Emi1 distinct from broader proliferation markers such as Ki67, cyclin A, and Bcl-2 in malignant neural tumors and lymphomas. The pink rectangles on the right vertical axes represent Emi1-positive tumors, which are largely malignant and positive for both proliferation and APC/C clusters. The yellow rectangles indicate mostly malignant tumors that are largely proliferation and APC/C cluster positive but Emi1 negative. The blue rectangles represent mostly benign or low-grade tumors that are predominantly proliferation cluster positive and APC/C cluster negative. ALCL, anaplastic large cell lymphoma; DLBCL, diffuse large B-cell lymphoma; FL1–3, follicular lymphoma grades 1 through 3; NKC, natural killer cell (lymphoma); NLP, nodular lymphocyte predominant Hodgkin’s lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; PTLD, posttransplant lymphoproliferative disorder.
Figure 6
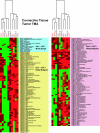
Hierarchical clustering analysis of Emi1, proliferation control, and mitotic control proteins in human connective tissue tumors. Data are represented as in Figure 4. DFSP, dermatofibrosarcoma protuberans; DSRCT, desmoplastic small round cell tumor; GIST, gastrointestinal stromal cell tumor; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; NOS, type not otherwise specified; PNET, primitive neuroectodermal tumor; SFT, solitary fibrous tumor.
Similar articles
- Early mitotic inhibitor-1, an anaphase-promoting complex/cyclosome inhibitor, can control tumor cell proliferation in hepatocellular carcinoma: correlation with Skp2 stability and degradation of p27(Kip1).
Zhao Y, Tang Q, Ni R, Huang X, Wang Y, Lu C, Shen A, Wang Y, Li C, Yuan Q, Chen H, Cheng C, He S. Zhao Y, et al. Hum Pathol. 2013 Mar;44(3):365-73. doi: 10.1016/j.humpath.2012.03.030. Epub 2012 Sep 17. Hum Pathol. 2013. PMID: 22995332 - The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells.
Engelbert D, Schnerch D, Baumgarten A, Wäsch R. Engelbert D, et al. Oncogene. 2008 Feb 7;27(7):907-17. doi: 10.1038/sj.onc.1210703. Epub 2007 Aug 13. Oncogene. 2008. PMID: 17700535 - APC/C Cdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase.
Floyd S, Pines J, Lindon C. Floyd S, et al. Curr Biol. 2008 Nov 11;18(21):1649-58. doi: 10.1016/j.cub.2008.09.058. Epub 2008 Oct 30. Curr Biol. 2008. PMID: 18976910 - Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis.
Eckerdt F, Strebhardt K. Eckerdt F, et al. Cancer Res. 2006 Jul 15;66(14):6895-8. doi: 10.1158/0008-5472.CAN-06-0358. Cancer Res. 2006. PMID: 16849530 Review. - Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells.
Wäsch R, Engelbert D. Wäsch R, et al. Oncogene. 2005 Jan 6;24(1):1-10. doi: 10.1038/sj.onc.1208017. Oncogene. 2005. PMID: 15637585 Review.
Cited by
- Human papillomavirus type 16 E7 oncoprotein inhibits the anaphase promoting complex/cyclosome activity by dysregulating EMI1 expression in mitosis.
Yu Y, Munger K. Yu Y, et al. Virology. 2013 Nov;446(1-2):251-9. doi: 10.1016/j.virol.2013.08.013. Epub 2013 Sep 5. Virology. 2013. PMID: 24074588 Free PMC article. - The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo.
Alkassar M, Gärtner B, Roemer K, Graesser F, Rommelaere J, Kaestner L, Haeckel I, Graf N. Alkassar M, et al. J Neurooncol. 2011 Sep;104(3):715-27. doi: 10.1007/s11060-011-0606-5. Epub 2011 May 24. J Neurooncol. 2011. PMID: 21607667 - Deregulation of F-box proteins and its consequence on cancer development, progression and metastasis.
Heo J, Eki R, Abbas T. Heo J, et al. Semin Cancer Biol. 2016 Feb;36:33-51. doi: 10.1016/j.semcancer.2015.09.015. Epub 2015 Sep 30. Semin Cancer Biol. 2016. PMID: 26432751 Free PMC article. Review. - Activation of the Anaphase Promoting Complex Reverses Multiple Drug Resistant Cancer in a Canine Model of Multiple Drug Resistant Lymphoma.
Arnason TG, MacDonald-Dickinson V, Gaunt MC, Davies GF, Lobanova L, Trost B, Gillespie ZE, Waldner M, Baldwin P, Borrowman D, Marwood H, Vizeacoumar FS, Vizeacoumar FJ, Eskiw CH, Kusalik A, Harkness TAA. Arnason TG, et al. Cancers (Basel). 2022 Aug 30;14(17):4215. doi: 10.3390/cancers14174215. Cancers (Basel). 2022. PMID: 36077749 Free PMC article. - The onconeural antigen cdr2 is a novel APC/C target that acts in mitosis to regulate c-myc target genes in mammalian tumor cells.
O'Donovan KJ, Diedler J, Couture GC, Fak JJ, Darnell RB. O'Donovan KJ, et al. PLoS One. 2010 Apr 7;5(4):e10045. doi: 10.1371/journal.pone.0010045. PLoS One. 2010. PMID: 20383333 Free PMC article.
References
- Cahill DP, Kinzler KW, Vogelstein B, Lengauer C. Genetic instability and Darwinian selection in tumours. Trends Cell Biol. 1999;9:M57–M60. - PubMed
- Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. - PubMed
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. - PubMed
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 NS045077/NS/NINDS NIH HHS/United States
- R01 GM060439/GM/NIGMS NIH HHS/United States
- K08 NS45077/NS/NINDS NIH HHS/United States
- R01 GM60439/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous