Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression - PubMed (original) (raw)
Comparative Study
Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression
Craig A Schiltz et al. BMC Biol. 2007.
Abstract
Background: Cues predictive of food availability are powerful modulators of appetite as well as food-seeking and ingestive behaviors. The neurobiological underpinnings of these conditioned responses are not well understood. Monitoring regional immediate early gene expression is a method used to assess alterations in neuronal metabolism resulting from upstream intracellular and extracellular signaling. Furthermore, assessing the expression of multiple immediate early genes offers a window onto the possible sequelae of exposure to food cues, since the function of each gene differs. We used immediate early gene and proenkephalin expression as a means of assessing food cue-elicited regional activation and alterations in functional connectivity within the forebrain.
Results: Contextual cues associated with palatable food elicited conditioned motor activation and corticosterone release in rats. This motivational state was associated with increased transcription of the activity-regulated genes homer1a, arc, zif268, ngfi-b and c-fos in corticolimbic, thalamic and hypothalamic areas and of proenkephalin within striatal regions. Furthermore, the functional connectivity elicited by food cues, as assessed by an inter-regional multigene-expression correlation method, differed substantially from that elicited by neutral cues. Specifically, food cues increased cortical engagement of the striatum, and within the nucleus accumbens, shifted correlations away from the shell towards the core. Exposure to the food-associated context also induced correlated gene expression between corticostriatal networks and the basolateral amygdala, an area critical for learning and responding to the incentive value of sensory stimuli. This increased corticostriatal-amygdalar functional connectivity was absent in the control group exposed to innocuous cues.
Conclusion: The results implicate correlated activity between the cortex and the striatum, especially the nucleus accumbens core and the basolateral amygdala, in the generation of a conditioned motivated state that may promote excessive food intake. The upregulation of a number of genes in unique patterns within corticostriatal, thalamic, and hypothalamic networks suggests that food cues are capable of powerfully altering neuronal processing in areas mediating the integration of emotion, cognition, arousal, and the regulation of energy balance. As many of these genes play a role in plasticity, their upregulation within these circuits may also indicate the neuroanatomic and transcriptional correlates of extinction learning.
Figures
Figure 1
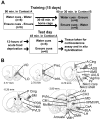
Schematic diagrams of the experimental paradigm and the brain regions analyzed for gene expression. (A) The experimental paradigm consisted of a 15-day training period in which all rats received access to chocolate Ensure and water in one of two distinct environments (contexts A and B). Locomotor activity was measured in context A over the training phase of the study. On the test day, 3 days after the final day of training, rats were reintroduced to context A after an acute 12-hour food deprivation period. Locomotor activity was measured in context A for 45 minutes, after which time rats were killed, and trunk blood and brains were taken for plasma corticosterone assay and in situ hybridization analysis of forebrain gene expression. (B) The brain regions analyzed for activity-dependent gene expression via in situ hybridization included the ventral orbital prefrontal cortex (VO), lateral orbital prefrontal cortex (LO), prelimbic cortex (PreL), infralimbic cortex (InfrL), anterior and posterior cingulate cortex (A and P Cing), primary sensory cortex (S1), agranular insular cortex (AgrIns), piriform cortex (Pir), sensorimotor cortex, nucleus accumbens shell (NAcc shell), nucleus accumbens core (NAcc core), anterior ventrolateral striatum (AVLS), anterior medial striatum (AMS), anterior dorsolateral striatum (ADLS), sensorimotor cortex (SM), posterior ventrolateral striatum (PVLS), posterior medial striatum (PMS), posterior dorsolateral striatum (PDLS), CA1 and CA3 subfields of the dorsal hippocampus, basolateral amygdala (BLA), central nucleus of the amygdala (CeA), periventricular thalamus (PVN), centromedian thalamus (CM), rhomboid and reunions thalamus (Rh/Re), mediodorsal thalamus (MD), ventralposteromedial and ventralposterolateral thalamus (VPM/VPL), lateral hypothalamus (LH), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and arcuate hypothalamus. Numbers represent distance from bregma in mm (adapted with permission from [131]).
Figure 2
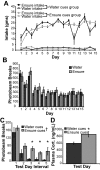
Behavioral and endocrine data. (A) Intake of Ensure and water by the water cues and Ensure cues groups in their respective contexts. Ensure intake was significantly greater than water intake and was not different between the groups (**p < 0.01). Ensure intake increased and reached a plateau over the 15 day training period (††p < 0.01). (B) Locomotor activity in response to access to water or Ensure in context A during daily training sessions. The levels of total horizontal activity between the groups did not differ significantly, and decreased during the training period, demonstrating that both groups habituated to context A despite different treatments in this environment. (C) While there was no difference in the patterned relationship of total horizontal activity with time between the groups, total horizontal activity was significantly higher on the test day in the Ensure cues group than in the water cues group during intervals 3, 4, and 5 (*p < 0.05). (D) Levels of plasma corticosterone in Ensure cues group were higher than in the water cues group (*p < 0.05).
Figure 3
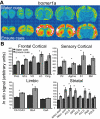
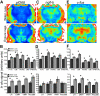
Telencephalic expression of homer1a induced by exposure to water or Ensure cues. (A) Pseudocolor autoradiographic phosphorescence images of coronal brain sections hybridized with a probe for homer1a from a rat in the water cues group (top) and the Ensure cues group (bottom). (B) Graphical representation of semiquantitative measurements of in situ hybridization for homer1a in telencephalic regions. Ensure cues increased the expression of homer1a in a many of the corticolimbic regions examined (*p < 0.05). For abbreviations, see legend to Figure 1.
Figure 4
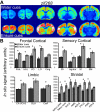
Telencephalic expression of zif268 induced by exposure to water or Ensure cues. (A) Pseudocolor autoradiographic phosphorescence images of coronal brain sections hybridized with a probe for zif268 from a rat in the water cues group (top) and the Ensure cues group (bottom). (B) Graphical representation of semiquantitative measurements of in situ hybridization for zif268 in telencephalic regions. Ensure cues increased the expression of zif268 in a many of the corticolimbic regions examined (*p < 0.05). For abbreviations, see legend to Figure 1.
Figure 5
Diencephalic expression of the transcription factor immediate early genes induced by exposure to water or Ensure cues. (A) Pseudocolor autoradiographic phosphorescence image of a coronal brain sections at the level of the diencephalons, hybridized with a probe for zif268 from a rat in the water cues group (top) and the Ensure cues group (bottom). (B) Graphical representation of semiquantitative measurements of in situ hybridization for zif268 in diencephalic regions. Ensure cues increased the expression of zif268 in thalamic and hypothalamic regions (*p < 0.05). (C) Pseudocolor autoradiographic phosphorescence images of coronal brain sections at the level of the diencephalon hybridized with a probe for ngfi-b from a rat in the water cues group (top) and the Ensure cues group (bottom). (D) Graphical representation of semiquantitative measurements of in situ hybridization for ngfi-b in diencephalic regions. Ensure cues increased the expression of ngfi-b in thalamic but not hypothalamic regions (*p < 0.05). (E) Pseudocolor autoradiographic phosphorescence image of a coronal brain sections at the level of the diencephalon hybridized with a probe for c-fos from a rat in the water cues group (top) and the Ensure cues group (bottom). (F) Graphical representation of semiquantitative measurements of in situ hybridization for c-fos in diencephalic regions. Ensure cues increased the expression of c-fos in thalamic and hypothalamic regions (*p < 0.05). For abbreviations, see legend to Figure 1.
Figure 6
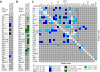
Graphical representation of the results of correlation analyses in the Ensure and water cues groups. Collapsed values for the expression of each of the immediate early genes that were normalized to regional control averages were used to calculate correlations within each group so long as there was a significant mean difference between the groups for each individual gene. Correlations that were significant are color-coded by the range in which their Pearson product moment value (r) lies (see key). Correlations that were not significant are indicated by light gray shading. (A) Correlations between regional immediate early gene expression and total horizontal activity in the water cues group (left column) and the Ensure cues group (right column). (B) Correlations between regional immediate early gene expression and plasma corticosterone in the water cues group (left column) and the Ensure cues group (right column). (C) Interregional gene expression correlations in the water cues group (lower left half) and the Ensure cues group (upper right half). NA designates a region for which correlations were not performed due to a lack of significant differences between the groups in all of the genes analyzed. The lack of interregional gene-expression correlations within the diencephalon most likely represents a decrease in the statistical power of the correlation analyses, as only a subset of the genes exhibited significant differences in expression within these regions. For abbreviations, see legend to Figure 1.
Figure 7
Striatal expression of hnPENK. (A) Pseudocolor autoradiographic phosphorescence image of a coronal brain sections at the level of the anterior (left) and posterior (right) striatum hybridized with a probe for hnPENK from a rat in the water cues group (top) and the Ensure cues group (bottom). (B) Graphical representation of semiquantitative measurements of in situ hybridization for hnPENK in striatal regions. Ensure cues increased the expression of hnPENK in the nucleus accumbens core and ventrolateral and medial regions, but not in the nucleus accumbens shell and dorsolateral regions (*p < 0.05). (C) Graphical representation of the results of extrastriatal immediate early gene expression and striatal hnPENK expression correlation analyses in both the water cues and the Ensure cues groups. Correlations that were significant are color-coded by the range in which their Pearson product moment (r) value lies (see key). Correlations that are not significant are shaded light gray. For abbreviations, see legend to Figure 1.
Figure 8
Circuit diagrams of the regions exhibiting interregional gene-expression correlations elicited by food cues (right) or water cues (left). Grey arrows represent relatively fewer numbers of interregional gene-expression correlations and black arrows represent relatively greater numbers of these correlations. Intracortical and intrastriatal interregional gene-expression correlations predominated in the water cues group. In this group, there were relatively few corticostriatal correlations, and cortical engagement of the shell of the nucleus accumbens was greater than that of the core subregion. The water cues group also exhibited correlated gene expression between the hippocampus and the basolateral amygdala. In contrast, the number of intracortical and intrastriatal correlations was relatively smaller and the number of corticostriatal correlations was relatively higher in the food cues group. There was also a shift of cortical correlations to the core of the nucleus accumbens in rats exposed to food cues. Another striking difference between the groups included the loss of the correlated gene expression between the hippocampus and the basolateral amygdala. The basolateral amygdala in the food cues group exhibited a great degree of correlated immediate early gene expression with the cortex and the striatum. These observations suggest that shifts in correlated activity between the cortex and striatum, particularly the nucleus accumbens core, and the basolateral amygdala are involved in the generation of a motivated state by salient external cues. For abbreviations, see legend to Figure 1.
Similar articles
- Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain.
Schiltz CA, Kelley AE, Landry CF. Schiltz CA, et al. Eur J Neurosci. 2005 Mar;21(6):1703-11. doi: 10.1111/j.1460-9568.2005.04001.x. Eur J Neurosci. 2005. PMID: 15845097 Free PMC article. - Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry.
Christiansen AM, Herman JP, Ulrich-Lai YM. Christiansen AM, et al. Stress. 2011 Mar;14(2):205-15. doi: 10.3109/10253890.2010.531331. Stress. 2011. PMID: 21291318 Free PMC article. - Upregulation of forebrain proenkephalin mRNA subsequent to NMDA receptor blockade.
Angulo JA, Watanabe Y, Cadet J, Ledoux M, McEwen BS. Angulo JA, et al. Eur J Pharmacol. 1993 Feb 15;244(3):317-8. doi: 10.1016/0922-4106(93)90158-6. Eur J Pharmacol. 1993. PMID: 8458404 - Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning.
Kelley AE. Kelley AE. Neurosci Biobehav Rev. 2004 Jan;27(8):765-76. doi: 10.1016/j.neubiorev.2003.11.015. Neurosci Biobehav Rev. 2004. PMID: 15019426 Review. - Pharmacologic regulation of striatal proenkephalin gene expression via transcription factor CREB.
Hyman SE, Konradi C, Kobierski L, Cole R, Senatus P, Green D. Hyman SE, et al. Prog Clin Biol Res. 1994;390:155-71. Prog Clin Biol Res. 1994. PMID: 7724644 Review. No abstract available.
Cited by
- Characterization of the transporterB0AT3 (Slc6a17) in the rodent central nervous system.
Hägglund MG, Hellsten SV, Bagchi S, Ljungdahl A, Nilsson VC, Winnergren S, Stephansson O, Rumaks J, Svirskis S, Klusa V, Schiöth HB, Fredriksson R. Hägglund MG, et al. BMC Neurosci. 2013 May 14;14:54. doi: 10.1186/1471-2202-14-54. BMC Neurosci. 2013. PMID: 23672601 Free PMC article. - Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice.
Davis JF, Choi DL, Clegg DJ, Benoit SC. Davis JF, et al. Physiol Behav. 2011 Apr 18;103(1):39-43. doi: 10.1016/j.physbeh.2010.10.017. Epub 2010 Oct 29. Physiol Behav. 2011. PMID: 21036184 Free PMC article. - Deep brain stimulation in rats: different targets induce similar antidepressant-like effects but influence different circuits.
Hamani C, Amorim BO, Wheeler AL, Diwan M, Driesslein K, Covolan L, Butson CR, Nobrega JN. Hamani C, et al. Neurobiol Dis. 2014 Nov;71:205-14. doi: 10.1016/j.nbd.2014.08.007. Epub 2014 Aug 13. Neurobiol Dis. 2014. PMID: 25131446 Free PMC article. - Loss of Function of Phosphodiesterase 11A4 Shows that Recent and Remote Long-Term Memories Can Be Uncoupled.
Pilarzyk K, Klett J, Pena EA, Porcher L, Smith AJ, Kelly MP. Pilarzyk K, et al. Curr Biol. 2019 Jul 22;29(14):2307-2321.e5. doi: 10.1016/j.cub.2019.06.018. Epub 2019 Jul 11. Curr Biol. 2019. PMID: 31303492 Free PMC article. - Differential modulation of arcuate nucleus and mesolimbic gene expression levels by central leptin in rats on short-term high-fat high-sugar diet.
van den Heuvel JK, Eggels L, Fliers E, Kalsbeek A, Adan RA, la Fleur SE. van den Heuvel JK, et al. PLoS One. 2014 Jan 31;9(1):e87729. doi: 10.1371/journal.pone.0087729. eCollection 2014. PLoS One. 2014. PMID: 24498181 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA004788/DA/NIDA NIH HHS/United States
- F30 DA016503/DA/NIDA NIH HHS/United States
- DA04788/DA/NIDA NIH HHS/United States
- R37 DA004788/DA/NIDA NIH HHS/United States
- DA016503/DA/NIDA NIH HHS/United States
- DA13780/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials