PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism - PubMed (original) (raw)
Comparative Study
doi: 10.1371/journal.pgen.0030064.
Sarah L Gray, Laxman Yetukuri, Kenju Shimomura, Sam Virtue, Mark Campbell, R Keira Curtis, Mercedes Jimenez-Linan, Margaret Blount, Giles S H Yeo, Miguel Lopez, Tuulikki Seppänen-Laakso, Frances M Ashcroft, Matej Oresic, Antonio Vidal-Puig
Affiliations
- PMID: 17465682
- PMCID: PMC1857730
- DOI: 10.1371/journal.pgen.0030064
Comparative Study
PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism
Gema Medina-Gomez et al. PLoS Genet. 2007.
Abstract
Peroxisome proliferator activated receptor gamma 2 (PPARg2) is the nutritionally regulated isoform of PPARg. Ablation of PPARg2 in the ob/ob background, PPARg2(-/-) Lep(ob)/Lep(ob) (POKO mouse), resulted in decreased fat mass, severe insulin resistance, beta-cell failure, and dyslipidaemia. Our results indicate that the PPARg2 isoform plays an important role, mediating adipose tissue expansion in response to positive energy balance. Lipidomic analyses suggest that PPARg2 plays an important antilipotoxic role when induced ectopically in liver and muscle by facilitating deposition of fat as relatively harmless triacylglycerol species and thus preventing accumulation of reactive lipid species. Our data also indicate that PPARg2 may be required for the beta-cell hypertrophic adaptive response to insulin resistance. In summary, the PPARg2 isoform prevents lipotoxicity by (a) promoting adipose tissue expansion, (b) increasing the lipid-buffering capacity of peripheral organs, and (c) facilitating the adaptive proliferative response of beta-cells to insulin resistance.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
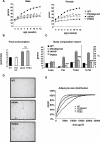
Figure 1. Physiological Characterisation of POKO Mouse
(A) Body weights (black circles, WT; black squares, ob/ob; white circles, PPARg2 KO; white squares, POKO) are shown for males (left) or females (right) (n = 5–12). *, p < 0.05 POKO versus ob/ob and §, p < 0.01 POKO versus WT. (B) Food intake from 20-wk-old female mice (n = 4) is shown. (C) Body composition analysis from 20-wk-old females is shown: WT, ob/ob, PPARg2 KO, and POKO mice fed chow diet mice (n = 4–7). *, p < 0.05 POKO versus WT and ###, p < 0.001 POKO versus ob/ob. (D) Haematoxylin and eosin (H and E)-stained sections (10×) from epididymal WAT from 16-wk-old male WT, ob/ob, and POKO mice. (E) Percent relative cumulative frequency analysis (PRCF) from epididymal WAT adipocytes from 16-wk-old male WT, ob/ob, PPARg2 KO, and POKO mice. (n = 4–5).
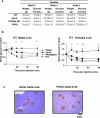
Figure 2. Early Insulin Resistance in POKO Mice Independent of Body Weight
(A) Body weight and plasma glucose levels from three, four, and five-week-old female WT, ob/ob, PPARg2 KO, and POKO. *, p < 0.05; **, p < 0.01; ***, p < 0.001 POKO versus ob/ob. (B) Plasma glucose levels during ITT on 4-wk-old male (left) and female (right) mice on chow diet (black triangle, WT; white triangle, PPARg2 KO; black square, ob/ob; black diamond, POKO) (n = 7). *, p < 0.05; **, p < 0.01 POKO versus ob/ob. (C) Morphological analysis of H and E-stained sections (10×) in pancreas from 4-wk-old males ob/ob and POKO mice (n = 5).
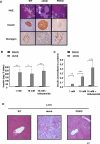
Figure 3. Impaired β-Cell Function and Hepatic Morphological Analyis in the POKO Mice
(A) H and E-stained sections (10×) and immunohistochemical (20×) analysis of insulin and glucagon in pancreas from 16-wk-old males WT, ob/ob, and POKO mice (n = 5). (B) Insulin content of islets isolated from POKO (black bars), and ob/ob (grey bars) mice. Each data point is the mean of six samples each of five islets. (C) Insulin secretion from islets isolated from POKO (black bars) and ob/ob (grey bars) mice in response to glucose (1, 16 mM) or glucose 16 mM + tolbutamide (200 μM). Data were collected from six samples each of five islets from three mice of each genotype. For each sample, insulin release was normalised to insulin content. *, p < 0.05; **, p < 0.01; ***, p < 0.001 POKO versus ob/ob. (D) H and E-stained sections (4×) in liver from 16-wk-old males WT, ob/ob, and POKO mice (n = 5).
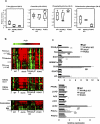
Figure 4. Lipidomic and Gene Expression Analysis of POKO WAT
(A) Lipidomic profiling of WAT from 16-wk-old males WT, ob/ob, and POKO mice. (B) Adipose tissue mRNA levels from different genes from 16-wk-old male WT, PPARg2 KO, ob/ob, and POKO mice (n = 6–8). *, p < 0.05; **, p <0.01; ***, p <0.001 POKO versus ob/ob.
Figure 5. Lipidomic and Gene Expression Analysis in Islets and Liver from POKO Mice
Lipidomic profiling of islets (A) and liver (B) from 16-wk-old males WT, PPARg2 KO, ob/ob, and POKO mice. TG, TAGs; DAGs, diacylglycerols; SM, sphingomyelins. (C) Liver gene expression from 16-wk-old male WT, ob/ob, PPARg2 KO, and POKO mice fed chow (n = 6–8). *, p < 0.05; **, p, <0.01; ***, p <0.001 POKO versus ob/ob; ###, p < 0.001 POKO versus WT; ‡‡‡, p < 0.001 ob/ob versus WT.
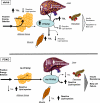
Figure 6. Storage of Lipids—Antilipotoxic Role of PPARg2
Antilipotoxic role of PPARg2 mediated by (a) expansion of adipose tissue and facilitation of triglyceride deposition and (b) facilitating deposition of fat in liver, skeletal muscle, and pancreas in the form of TAG. Ob/Ob mice can induce PPARg2 expression in liver, muscle, and β-cell, facilitating deposition of excess of energy in these organs in the form of TAG. Absence of inducibility of PPARg2 in POKO mouse liver, muscle, and β-cells results in increased deposition of reactive lipid species and decreased TAG, leading to marked insulin resistance and β-cell failure.
Similar articles
- Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice.
Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O'Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Gray SL, et al. Diabetes. 2006 Oct;55(10):2669-77. doi: 10.2337/db06-0389. Diabetes. 2006. PMID: 17003330 - Role of PPARg2 transcription factor in thiazolidinedione-induced insulin sensitization.
Saraf N, Sharma PK, Mondal SC, Garg VK, Singh AK. Saraf N, et al. J Pharm Pharmacol. 2012 Feb;64(2):161-71. doi: 10.1111/j.2042-7158.2011.01366.x. Epub 2011 Oct 13. J Pharm Pharmacol. 2012. PMID: 22221092 Review. - Early peroxisome proliferator-activated receptor gamma regulated genes involved in expansion of pancreatic beta cell mass.
Vivas Y, Martínez-García C, Izquierdo A, Garcia-Garcia F, Callejas S, Velasco I, Campbell M, Ros M, Dopazo A, Dopazo J, Vidal-Puig A, Medina-Gomez G. Vivas Y, et al. BMC Med Genomics. 2011 Dec 30;4:86. doi: 10.1186/1755-8794-4-86. BMC Med Genomics. 2011. PMID: 22208362 Free PMC article. - Sex dimorphic actions of rosiglitazone in generalised peroxisome proliferator-activated receptor-gamma (PPAR-gamma)-deficient mice.
Duan SZ, Usher MG, Foley EL 4th, Milstone DS, Brosius FC 3rd, Mortensen RM. Duan SZ, et al. Diabetologia. 2010 Jul;53(7):1493-505. doi: 10.1007/s00125-010-1748-2. Epub 2010 Apr 17. Diabetologia. 2010. PMID: 20401461 Free PMC article. - Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1).
Medina-Gomez G, Gray S, Vidal-Puig A. Medina-Gomez G, et al. Public Health Nutr. 2007 Oct;10(10A):1132-7. doi: 10.1017/S1368980007000614. Public Health Nutr. 2007. PMID: 17903321 Review.
Cited by
- Hepatic Peroxisome Proliferator-Activated Receptor Gamma Signaling Contributes to Alcohol-Induced Hepatic Steatosis and Inflammation in Mice.
Zhang W, Sun Q, Zhong W, Sun X, Zhou Z. Zhang W, et al. Alcohol Clin Exp Res. 2016 May;40(5):988-99. doi: 10.1111/acer.13049. Epub 2016 Apr 8. Alcohol Clin Exp Res. 2016. PMID: 27062444 Free PMC article. - Cladosporols and PPARγ: Same Gun, Same Bullet, More Targets.
Rapuano R, Mercuri A, Dallavalle S, Moricca S, Lavecchia A, Lupo A. Rapuano R, et al. Biomolecules. 2024 Aug 13;14(8):998. doi: 10.3390/biom14080998. Biomolecules. 2024. PMID: 39199386 Free PMC article. Review. - Elimination of the CDP-ethanolamine pathway disrupts hepatic lipid homeostasis.
Leonardi R, Frank MW, Jackson PD, Rock CO, Jackowski S. Leonardi R, et al. J Biol Chem. 2009 Oct 2;284(40):27077-89. doi: 10.1074/jbc.M109.031336. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666474 Free PMC article. - Inflammation and Obesity: The Pharmacological Role of Flavonoids in the Zebrafish Model.
Russo C, Maugeri A, Musumeci L, De Sarro G, Cirmi S, Navarra M. Russo C, et al. Int J Mol Sci. 2023 Feb 2;24(3):2899. doi: 10.3390/ijms24032899. Int J Mol Sci. 2023. PMID: 36769222 Free PMC article. Review. - CoA protects against the deleterious effects of caloric overload in Drosophila.
Palanker Musselman L, Fink JL, Baranski TJ. Palanker Musselman L, et al. J Lipid Res. 2016 Mar;57(3):380-7. doi: 10.1194/jlr.M062976. Epub 2016 Jan 24. J Lipid Res. 2016. PMID: 26805007 Free PMC article.
References
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. - PubMed
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. - PubMed
- Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes. 2001;2:S189–S201. 109 Suppl. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous