Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells - PubMed (original) (raw)
Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells
T Albrektsen et al. J Thromb Haemost. 2007 Aug.
Abstract
Background: Factor VIIa (FVIIa) binding to tissue factor (TF) induces cell signaling via the protease activity of FVIIa and protease-activated receptor 2 (PAR2).
Objective: We examined how the gene-expression profile induced by FVIIa corresponds to the profiles induced by protease-activated receptor 1 (PAR1) or PAR2 agonists using MDA-MB-231 breast carcinoma cells that constitutively express TF, PAR1 and PAR2.
Results and conclusions: Out of 8500 genes, FVIIa stimulation induced differential regulation of 39 genes most of which were not previously recognized as FVIIa regulated. All genes regulated by FVIIa were similarly regulated by a PAR2 agonist peptide confirming FVIIa signaling via PAR2. An appreciable fraction of the PAR2-regulated genes was also regulated by a PAR1 agonist peptide suggesting extensive redundancy between FVIIa/PAR2 signaling and thrombin/PAR1 signaling. The FVIIa regulated genes encode cytokines, chemokines and growth factors, and the gene repertoire induced by FVIIa in MDA-MB-231 cells is consistent with a role for TF-FVIIa signaling in regulation of a wound healing type of response. Interestingly, a number of genes regulated exclusively by FVIIa/PAR2-mediated cell signaling in MDA-MB-231 cells were regulated by thrombin and a PAR1 agonist, but not by FVIIa, in the TF-expressing glioblastoma U373 cell line.
Figures
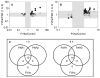
Fig. 1
cDNA microarray analysis comparing transcriptional effects induced by factor VIIa (FVIIa) and selective protease-activated receptor 1 (PAR1) and PAR2 agonist peptides in MDA-MB-231 cells. Quiescent MDA-MB-231 cells were treated for 1 (A) or 6 h (B) with serum-free control media, or control media supplemented with 100 μ
m
FVIIa, 10 μ
m
TFLLRNPNDK (PAR1 agonist peptide) or 50 μ
m
SLIGKV (PAR2 agonist peptide). Genes significantly (P < 0.01) differentially regulated more than 2-fold by FVIIa are included. The differential gene regulation ratio of FVIIa/PAR1 (squares) or the ratio of FVIIa/PAR2 (triangles) is plotted against the FVIIa/media control ratio. FVIIa/PAR1 and FVIIa/PAR2 ratios below 2-fold are to be found within the grey-scaled areas and represent genes regulated similarly by FVIIa and the PAR agonist peptides. Data points present in white-scaled areas with FVIIa/PAR1 or FVIIa/PAR2 ratios higher than 2-fold represent genes regulated by FVIIa but not the PAR1 agonist peptide (squares), or by FVIIa but not the PAR2 agonist peptide (triangles). Panel C is a schematic representation of cDNA microarray results comparing transcriptional effects induced by FVIIa and PAR1 and PAR2 agonist peptides.
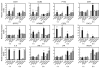
Fig. 2
Validation of factor VIIa (FVIIa)-induced differential gene expression in MDA-MB-231 cells by real time qPCR analysis. Total RNA samples used for cDNA micro-array analysis were subjected to real-time qPCR analysis. Regulation of the mRNA level was measured relative to media control (mean ± SEM; n = 3). Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was determined by _t_-test analysis.
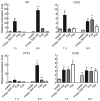
Fig. 3
Differential gene regulation by factor VIIa (FVIIa), FXa and thrombin in MDA-MB-231 cells. Total RNA from MDA-MB-231 cells treated with control media, or control media supplemented with FVIIa (10 n
m
) + Tick anticoagulant protein (100 n
m
) + hirudin (25 U mL−1), FXa (100 n
m
), or thrombin (FIIa) (10 n
m
) for 1 or 6 h was isolated and subjected to real-time qPCR analysis. Regulation of the mRNA level was measured relative to media control (mean ± SEM; n = 3). Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was determined by _t_-test analysis.
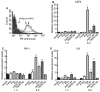
Fig. 4
Differential gene regulation in U373 cells. (A) Flow cytometry of U373 cells probed with antiprotease-activated receptor 1 (PAR1), anti-PAR2 or antitissue factor (TF). (B–D) Total RNA from U373 cells treated with: media; 50 n
m
FVIIa; 50 n
m
FXa; 50 n
m
thrombin (FIIa); 10 μ
m
PAR1 agonist peptide or 50 μ
m
PAR2 agonist peptide for 1 or 6 h was isolated and subjected to real-time qPCR analysis. Regulation of the mRNA level was measured relative to media control (mean ± SEM; n = 2). Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was determined by _t_-test analysis.
Comment in
- Redundant signaling of tissue factor and thrombin in cancer progression?
Ruf W. Ruf W. J Thromb Haemost. 2007 Aug;5(8):1584-7. doi: 10.1111/j.1538-7836.2007.02622.x. J Thromb Haemost. 2007. PMID: 17663729 No abstract available.
Similar articles
- Microarray studies of factor VIIa-activated cancer cells.
Petersen LC. Petersen LC. Thromb Res. 2008;122 Suppl 1:S11-3. doi: 10.1016/S0049-3848(08)70011-2. Thromb Res. 2008. PMID: 18691491 Review. - Engineering of substrate selectivity for tissue factor.factor VIIa complex signaling through protease-activated receptor 2.
Larsen KS, Ostergaard H, Olsen OH, Bjelke JR, Ruf W, Petersen LC. Larsen KS, et al. J Biol Chem. 2010 Jun 25;285(26):19959-66. doi: 10.1074/jbc.M110.101030. Epub 2010 Apr 13. J Biol Chem. 2010. PMID: 20388709 Free PMC article. - Tissue Factor/ FVIIa prevents the extrinsic pathway of apoptosis by regulation of the tumor suppressor Death-Associated Protein Kinase 1 (DAPK1).
Aberg M, Johnell M, Wickström M, Siegbahn A. Aberg M, et al. Thromb Res. 2011 Feb;127(2):141-8. doi: 10.1016/j.thromres.2010.11.015. Epub 2010 Dec 17. Thromb Res. 2011. PMID: 21168190 - The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors.
Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, Teyton L, Petersen LC, Ruf W. Disse J, et al. J Biol Chem. 2011 Feb 18;286(7):5756-67. doi: 10.1074/jbc.M110.201228. Epub 2010 Dec 13. J Biol Chem. 2011. PMID: 21149441 Free PMC article. - Role of tissue factor in cancer.
Kasthuri RS, Taubman MB, Mackman N. Kasthuri RS, et al. J Clin Oncol. 2009 Oct 10;27(29):4834-8. doi: 10.1200/JCO.2009.22.6324. Epub 2009 Sep 8. J Clin Oncol. 2009. PMID: 19738116 Free PMC article. Review.
Cited by
- Twist-mediated PAR1 induction is required for breast cancer progression and metastasis by inhibiting Hippo pathway.
Wang Y, Liao R, Chen X, Ying X, Chen G, Li M, Dong C. Wang Y, et al. Cell Death Dis. 2020 Jul 9;11(7):520. doi: 10.1038/s41419-020-2725-4. Cell Death Dis. 2020. PMID: 32647142 Free PMC article. - Platelets, coagulation and fibrinolysis in breast cancer progression.
Lal I, Dittus K, Holmes CE. Lal I, et al. Breast Cancer Res. 2013;15(4):207. doi: 10.1186/bcr3425. Breast Cancer Res. 2013. PMID: 23905544 Free PMC article. Review. - Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations.
Magnus N, Garnier D, Meehan B, McGraw S, Lee TH, Caron M, Bourque G, Milsom C, Jabado N, Trasler J, Pawlinski R, Mackman N, Rak J. Magnus N, et al. Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3544-9. doi: 10.1073/pnas.1314118111. Epub 2014 Feb 11. Proc Natl Acad Sci U S A. 2014. PMID: 24520174 Free PMC article. - Brain neoplasms and coagulation-lessons from heterogeneity.
D'Asti E, Fang Y, Rak J. D'Asti E, et al. Rambam Maimonides Med J. 2014 Oct 29;5(4):e0030. doi: 10.5041/RMMJ.10164. eCollection 2014 Oct. Rambam Maimonides Med J. 2014. PMID: 25386346 Free PMC article. - Tissue factor and cancer.
Ruf W. Ruf W. Thromb Res. 2012 Oct;130 Suppl 1(0 1):S84-7. doi: 10.1016/j.thromres.2012.08.285. Thromb Res. 2012. PMID: 23026674 Free PMC article. Review.
References
- Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. - PubMed
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22. - PubMed
- Taubman MB, Fallon JT, Schecter AD, Giesen P, Mendlowitz M, Fyfe BS, Marmur JD, Nemerson Y. Tissue factor in the pathogenesis of atherosclerosis. Thromb Haemost. 1997;78:200–4. - PubMed
- Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL065500-06/HL/NHLBI NIH HHS/United States
- R01 HL065500-05A2/HL/NHLBI NIH HHS/United States
- R01 HL065500/HL/NHLBI NIH HHS/United States
- HL65550/HL/NHLBI NIH HHS/United States
- R01 HL058869-07/HL/NHLBI NIH HHS/United States
- R01 HL058869/HL/NHLBI NIH HHS/United States
- HL58869/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous