Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease - PubMed (original) (raw)
Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease
Xiaofeng Gu et al. Mol Neurodegener. 2007.
Abstract
A critical issue in understanding Huntington's disease (HD) pathogenesis is how the ubiquitously expressed mutant huntingtin (mhtt) with an expanded polyglutamine repeat can cause selective toxicity of striatal and cortical neurons. Two potential cellular models may contribute to such specificity: expression of mhtt in these vulnerable neurons alone may be sufficient to result in their dysfunction and/or degeneration (cell-autonomous model); or mhtt in other cell types can elicit pathological cell-cell interactions to cause the vulnerable neurons to become dysfunctional and be at risk for degeneration (cell-cell interaction model). To distinguish between these two models, we have selectively expressed a neuropathogenic fragment of mhtt-exon1 in striatal medium spiny neurons (MSNs) by crossing a conditional mouse model of HD with a striatal-specific Cre mouse line. In this striatal model of HD, we observed progressive and cell-autonomous nuclear accumulation of mhtt aggregates in MSNs. Surprisingly, unlike the mouse model expressing mhtt-exon1 in all the neurons in the brain, the striatal model lacks significant locomotor deficits and striatal neuropathology including gliosis and dark degenerating neurons. Electrophysiological findings from acutely dissociated MSNs revealed a cell-autonomous deficit in N-methyl-d-aspartate (NMDA) receptor sensitivity to Mg2+, a deficit also present in other mouse models of HD. In conclusion, this study provides the first in vivo genetic evidence that pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of HD, and suggests a ''two-hit'' hypothesis in which both cell-autonomous toxicity and pathological cell-cell interactions are critical to HD pathogenesis.
Figures
Figure 1
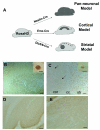
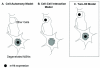
Generation of the striatal specific mouse model of HD. (A) Schematics illustrate the strategy to use cell-type-specific Cre to selectively activate mhtt-exon1 expression in all neurons in the brain (pan-neuronal model), only in CPNs (cortical model; [27]), and only in striatal MSNs and a subset of interneurons (asterisks) in the cortex (striatal model). (B) Immunohistochemical staining using polyclonal EM48 antibody, which is specific to aggregated forms of mhtt [9], reveals a cell-autonomous accumulation of nuclear mhtt aggregates in the striatum of the striatal model at 6 months of age. Inset in B shows at a higher magnification the EM48 staining in the nucleus is consisted of diffuse nuclear staining, and not large nuclear inclusions or smaller microaggregates. (C) EM48 staining reveal strong nuclear staining in the striatum (str) but sparse nuclear staining in the cortex (cor), inset in C shows diffuse EM48 nuclear staining of one cell at high magnification. cc: corpus callosum. Finally, there is no nuclear EM48 staining in other brain regions including cerebellum (D) and brain stem (E). Scale bars are 50 μm except in B (200 μm) and insets in B and C (10 μm).
Figure 2
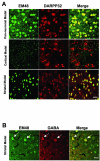
Selective accumulation of mhtt aggregates in MSNs in the striatal model. (A) Double immunofluorescent staining with EM48 antibody [30] and anti-Darpp32 (a MSN marker) using 6 month old coronal brain sections from striatal, cortical, and pan-neuronal models of HD. In both striatal and pan-neuronal models the vast majority of MSNs (>98%) have diffuse nuclear EM48 staining. Such a staining pattern is not present in the MSNs in the cortical model or WT mice at this age (data not shown for the WT). (B). Double immunofluorescent staining with EM48 antibody and anti-GABA antibody (marker for cortical interneurons) in the striatal model of HD reveal that a few cells that accumulate nuclear EM48 staining in the cortex are GABA (+) interneurons (white arrows). Scale bars: 100 μm
Figure 3
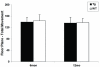
Lack of progressive locomotor deficits in the striatal model. In the striatal model, locomotor activity in the open field test is normal at 6 and 12 months, demonstrating cell-autonomous accumulation of mhtt aggregates in MSNs are not sufficient to elicit a motor deficit.
Figure 4
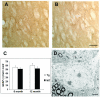
Lack of robust neuropathology in the striatal model. In the striatal model, we did not detect gliosis as indicated by the lack of GFAP staining in 12 month old transgenic mice (A) and in WT mice (12 months, B). Quantification of striatal gliosis [27] did not reveal any statistically significant differences between the two genotypes (C). (D) EM analyses did not reveal any degenerating dark neurons in the striatum (N = 2 per genotype). A representative healthy striatal neuron from the striatal model at 12 months of age is shown. Scale bars: A and B 100 μm; D 5 μm.
Figure 5
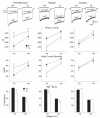
Alterations in NMDA receptor-mediated currents. Top panel. Representative traces of currents induced by 100 μM NMDA in the presence or absence of 50 μM Mg2+ at a holding potential of -70 mV. In the pan-neuronal model, the NMDA current in the absence of Mg2+ is smaller in the transgenic (Tg) cell while in the presence of Mg2+, the current is similar to the WT. In the striatal model, the NMDA current in the presence or absence of Mg2+ is larger in the transgenic cell than in the WT cell. In the cortical model NMDA currents in the presence or absence of Mg2+ were similar in WT and transgenic cells. 2nd and 3rd Panels. Graphs showing mean peak currents (± SEM) and mean peak current densities (± SEM) evoked by 100 μM NMDA in the absence of Mg2+ in each model. In the pan-neuronal model, NMDA peak currents were significantly smaller in transgenic cells at -70 and -40 mV and current densities were significantly smaller at -70 mV only. In contrast, in the striatal model, NMDA peak currents were significantly larger in transgenic cells at -70 and -40 mV and current densities were significantly larger at -70 mV only. In the cortical model, there were no differences in NMDA peak currents and current densities between WT and transgenic cells at any holding potential. Bottom Panel. Bar graphs showing mean percent block (± SEM) of NMDA currents by 50 μM Mg2+. In the pan-neuronal and striatal models, Mg2+ block was significantly smaller in transgenic compared to WT cells at -70 and -40 mV. In the cortical model, there were no differences in Mg2+ block between WT and transgenic cells at any holding potential.
Figure 6
Schematics illustrating the two-hit hypothesis of neuropathogenesis in conditional models of HD. Our current analyses of the striatal model and our previous study of the cortical and pan-neuronal model of HD have shown that behavioral deficits and robust cortical and striatal neuropathology are observed only when mhtt-exon1 is broadly expressed in the brain (pan-neuronal model). When mhtt-exon1 only is expressed in one of the known vulnerable neuronal populations, CPNs (cortical model) or striatal MSNs (striatal model), significant motor deficits or robust gliosis and degenerative changes did not occur despite cell-autonomous accumulation of mhtt aggregates. Thus, these findings rule out the cell-autonomous model which suggests expression of mhtt in the vulnerable neurons alone is sufficient to elicit robust neuropathology in that neuron (A). Since in both the cortical model [27] and in the striatal model, cell-autonomous expression of mhtt did exhibit evidence of neuronal dysfunction (i.e. degenerating lysosomes in the cortical model and electrophysiological changes in striatal model), the findings also suggest a pure cell-cell interaction model (B) is also unlikely. Thus, our current data favor the two-hit hypothesis (C), which suggests both cell-autonomous toxicity and pathological cell-cell interaction synergistically contribute to neuropathogenesis of the vulnerable cortical and striatal neurons.
Similar articles
- Forebrain striatal-specific expression of mutant huntingtin protein in vivo induces cell-autonomous age-dependent alterations in sensitivity to excitotoxicity and mitochondrial function.
Kim SH, Thomas CA, André VM, Cummings DM, Cepeda C, Levine MS, Ehrlich ME. Kim SH, et al. ASN Neuro. 2011 Jun 7;3(3):e00060. doi: 10.1042/AN20110009. ASN Neuro. 2011. PMID: 21542802 Free PMC article. - Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice.
Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, Heintz N, Yang XW. Gu X, et al. Neuron. 2005 May 5;46(3):433-44. doi: 10.1016/j.neuron.2005.03.025. Neuron. 2005. PMID: 15882643 - Mutant huntingtin reduction in astrocytes slows disease progression in the BACHD conditional Huntington's disease mouse model.
Wood TE, Barry J, Yang Z, Cepeda C, Levine MS, Gray M. Wood TE, et al. Hum Mol Genet. 2019 Feb 1;28(3):487-500. doi: 10.1093/hmg/ddy363. Hum Mol Genet. 2019. PMID: 30312396 Free PMC article. - Functional interactions within striatal microcircuit in animal models of Huntington's disease.
Ghiglieri V, Bagetta V, Calabresi P, Picconi B. Ghiglieri V, et al. Neuroscience. 2012 Jun 1;211:165-84. doi: 10.1016/j.neuroscience.2011.06.075. Epub 2011 Jul 1. Neuroscience. 2012. PMID: 21756979 Review. - Mitochondria and Huntington's disease pathogenesis: insight from genetic and chemical models.
Browne SE. Browne SE. Ann N Y Acad Sci. 2008 Dec;1147:358-82. doi: 10.1196/annals.1427.018. Ann N Y Acad Sci. 2008. PMID: 19076457 Review.
Cited by
- Huntington's Disease and Striatal Signaling.
Roze E, Cahill E, Martin E, Bonnet C, Vanhoutte P, Betuing S, Caboche J. Roze E, et al. Front Neuroanat. 2011 Aug 23;5:55. doi: 10.3389/fnana.2011.00055. eCollection 2011. Front Neuroanat. 2011. PMID: 22007160 Free PMC article. - Forebrain striatal-specific expression of mutant huntingtin protein in vivo induces cell-autonomous age-dependent alterations in sensitivity to excitotoxicity and mitochondrial function.
Kim SH, Thomas CA, André VM, Cummings DM, Cepeda C, Levine MS, Ehrlich ME. Kim SH, et al. ASN Neuro. 2011 Jun 7;3(3):e00060. doi: 10.1042/AN20110009. ASN Neuro. 2011. PMID: 21542802 Free PMC article. - Molecular insights into cortico-striatal miscommunications in Huntington's disease.
Veldman MB, Yang XW. Veldman MB, et al. Curr Opin Neurobiol. 2018 Feb;48:79-89. doi: 10.1016/j.conb.2017.10.019. Epub 2017 Nov 7. Curr Opin Neurobiol. 2018. PMID: 29125980 Free PMC article. Review. - The chicken or the egg: mitochondrial dysfunction as a cause or consequence of toxicity in Huntington's disease.
Polyzos AA, McMurray CT. Polyzos AA, et al. Mech Ageing Dev. 2017 Jan;161(Pt A):181-197. doi: 10.1016/j.mad.2016.09.003. Epub 2016 Sep 12. Mech Ageing Dev. 2017. PMID: 27634555 Free PMC article. Review. - Caenorhabditis elegans as a model system for studying non-cell-autonomous mechanisms in protein-misfolding diseases.
Nussbaum-Krammer CI, Morimoto RI. Nussbaum-Krammer CI, et al. Dis Model Mech. 2014 Jan;7(1):31-9. doi: 10.1242/dmm.013011. Dis Model Mech. 2014. PMID: 24396152 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources