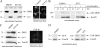Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death - PubMed (original) (raw)
Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death
Sylwia Wasiak et al. J Cell Biol. 2007.
Abstract
Dynamin-related protein 1 (DRP1) plays an important role in mitochondrial fission at steady state and during apoptosis. Using fluorescence recovery after photobleaching, we demonstrate that in healthy cells, yellow fluorescent protein (YFP)-DRP1 recycles between the cytoplasm and mitochondria with a half-time of 50 s. Strikingly, during apoptotic cell death, YFP-DRP1 undergoes a transition from rapid recycling to stable membrane association. The rapid cycling phase that characterizes the early stages of apoptosis is independent of Bax/Bak. However, after Bax recruitment to the mitochondrial membranes but before the loss of mitochondrial membrane potential, YFP-DRP1 becomes locked on the membrane, resulting in undetectable fluorescence recovery. This second phase in DRP1 cycling is dependent on the presence of Bax/Bak but independent of hFis1 and mitochondrial fragmentation. Coincident with Bax activation, we detect a Bax/Bak-dependent stimulation of small ubiquitin-like modifier-1 conjugation to DRP1, a modification that correlates with the stable association of DRP1 with mitochondrial membranes. Altogether, these data demonstrate that the apoptotic machinery regulates the biochemical properties of DRP1 during cell death.
Figures
Figure 1.
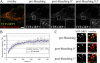
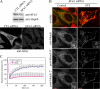
YFP-DRP1 rapidly cycles between the cytosol and preexisting sites on the mitochondrial membranes. (A) HeLa cells were transfected with YFP-DRP1 and Oct-DsRed (overlay). The boxed regions containing YFP-DRP1 staining (insets) associated with mitochondria was photobleached (0 min), and fluorescence recovery was monitored over time (8.5 min). Bar, 5 μm. (B) Relative fluorescence intensity of YFP-DRP1 recorded during a photobleaching protocol was plotted as a function of time (n = 7 cells). Equations used to calculate these parameters are described in Materials and methods. Error bars represent SD. (C) YFP-DRP1 (green) localized to mitochondria labeled with the MitoFluor red dye (red) was photobleached (0 min), and the fluorescence recovery was monitored over time (5 min). Arrowheads indicate recovery to the same puncta.
Figure 2.
The in vitro recruitment of recombinant DRP1 is not enhanced during apoptosis. (A) Cytosolic and mitochondrial fractions purified from sHeLa cells treated with DMSO or STS for 2 h were subjected to SDS-PAGE, transferred to nitrocellulose, and blotted with antibodies against DRP1 and cytochrome c (cyt c; left). Mitochondrial fractions were incubated at 37°C with the MitoFluor dye to monitor the presence of mitochondrial ΔΨ (right). (B) Mitochondrial fractions characterized in A were incubated with cytosol and GST-DRP1 for 1 h at the indicated temperatures. After the incubation, the reactions were centrifuged through a sucrose cushion to recover membranes, which were subsequently resolved by SDS-PAGE. Western blots revealed that GST-DRP1 and Tom20 associated with pelleted mitochondrial fractions. (C) Mitochondrial fractions purified from sHeLa cells treated with DMSO or STS for 4 h were subjected to SDS-PAGE, transferred to nitrocellulose, and blotted with antibodies against DRP1, cytochrome c (cyt c), and Tom20 (left). The same membrane fractions were incubated at 37°C with the MitoFluor red dye to monitor the presence of mitochondrial ΔΨ (right). (D) Mitochondrial fractions characterized in C were incubated at 37°C with cytosol purified from DMSO or STS-treated cells expressing GFP or YFP-DRP1. The reactions were centrifuged through a sucrose cushion to recover membrane-associated proteins, which were subsequently resolved by SDS-PAGE. Western blots revealed that YFP-DRP1 and Tom20 associated with mitochondrial fractions.
Figure 3.
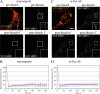
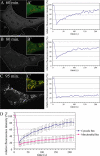
YFP-DRP1 fluorescence recovery to mitochondrial membranes is inhibited during apoptosis. (A and C) HeLa cells cotransfected with YFP-DRP1 and Oct-DsRed (overlay) were treated with STS or with anti-Fas–activating antibodies for the indicated amounts of time. The boxed regions (insets) were photobleached (0 min), and fluorescence recovery was monitored over time (5 min). (B and D) Relative fluorescence intensity of YFP-DRP1 recorded during a photobleaching protocol was plotted as a function of time. FRAP curves from16 cells treated with STS for 90–240 min (B) and from 15 cells treated with anti-Fas antibodies for 180–315 min (D) were used to generate mean plots. Error bars represent SD. Bar, 5 μm.
Figure 4.
The apoptotic block in YFP-DRP1 cycling occurs after fragmentation but before the loss of mitochondrial membrane potential. (A) Scatter plot representing maximal fluorescence recovery recorded during a FRAP protocol in 42 cells expressing YFP-DRP1 as a function of time of treatment with STS (blue diamonds). Pink circles represent the percentage of cells showing cytosolic cytochrome c as determined by indirect immunofluorescence at different times of STS treatment. Values are means of three independent experiments. Error bars represent SD. (B) A FRAP plot from a cell treated for 100 min with STS expressing YFP-DRP1 (green) and Oct-DsRed (red; B′). Mitochondria display a characteristic beads-on-a-string appearance, indicating active fragmentation (B′ and Video 1, available at
http://www.jcb.org/cgi/content/full/jcb.200610042/DC1
). (C and C′) A FRAP plot (C) from a cell treated for 120 min with STS expressing YFP-DRP1 and Oct-DsRed (green; C′). Fragmented mitochondria are labeled with MitoFluor red (red), indicating the presence of ΔΨ (C′). Video 2 illustrates the lack of mitochondrial movement after fragmentation. Bar, 5 μm.
Figure 5.
hFis1 is not required for YFP-DRP1 recycling at steady state or during apoptosis. (A) Control or specific siRNAs were used to silence the expression of hFis1 in HeLa cells, as shown by Western blotting and by indirect immunofluorescence with antibodies directed against hFis1. Antibodies against Hsp60 were used as a loading control (top). (B) hFis1 siRNA–treated Hela cells transfected with YFP-DRP1 were untreated or treated with 1 μM STS for >150 min. The overlay shows YFP-DRP1 (green) and a mitochondrial staining with MitoFluor red (red). Note the interconnected mitochondrial tubules in STS-treated cells. The boxed regions (insets) were photobleached (0 min), and fluorescence recovery was monitored over time (2 min). Bars, 5 μm. (C) Relative fluorescence intensity of YFP-DRP1 recorded during the photobleaching protocol was plotted as a function of time. FRAP curves from hFis1 siRNA–treated cells incubated with control media (n = 9) or with media containing STS for 150–250 min (n = 10) were used to generate mean plots. Error bars represent SD.
Figure 6.
The arrest in YFP-DRP1 cycling correlates with Bax translocation to the mitochondrial membrane. (A–C) HeLa cells coexpressing YFP-DRP1 and CFP-Bax were treated with STS for 20–120 min before photobleaching. The distribution of CFP-Bax in a representative cell is documented on the left, whereas the fluorescence recovery of YFP-DRP1 in the same cell is shown on the right. Insets (A′–C′) show colocalization between YFP-DRP1 (red) and CFP-Bax (green). The dotted lines in A and B show the outlines of one cell. (D) Averaged FRAP curves showing the cycling of YFP-DRP1 in cells displaying the cytosolic (triangles; n = 11) or mitochondrial (squares; n = 17) distribution of CFP-Bax treated with STS for 20–120 min. Error bars represent SD.
Figure 7.
The block in cycling of YFP-DRP1 during apoptosis is Bax/Bak dependent. (A) Wild-type (WT) and Bax/Bak double knockout (DKO) BMK cells were transfected with YFP-DRP1 and treated with STS for 195–365 min before photobleaching. The boxed regions containing YFP-DRP1 (green) associated with mitochondria (red) was photobleached (0 min), and fluorescence recovery was monitored over time (4.5 min; insets). Bar, 5 μm. (B) Averaged FRAP curves showing the cycling of YFP-DRP1 in WT (diamonds; n = 12) and Bax/Bak knockout cells (DKO; squares; n = 14) treated with STS for 195–365 min. Error bars represent SD.
Figure 8.
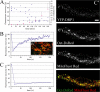
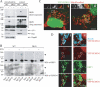
DRP1 is sumoylated during apoptosis in a Bax/Bak-dependent manner. (A) Mitochondrial and cytosolic fractions purified from BMK wild-type (WT) or Bax/Bak double knockout (DKO) cells treated with DMSO or STS for 4 h were subjected to SDS-PAGE, transferred to nitrocellulose, and blotted with antibodies against DRP1 and cytochrome c (cyt c), Bax, and Hsp60. Long exposure reveals a 150-kD band recognized by the anti-DRP antibody. (B) Total lysates from BMK WT and DKO cells were subjected to immunoprecipitation with total mouse IgG or anti-DRP1 antibodies. Specifically bound proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with antibodies against DRP1 and SUMO-1. Input represents 5% of the total lysate used for immunoprecipitation. Closed arrowheads point to bands at 150 kD that are immunoreactive for both DRP1 and SUMO-1. Open arrowhead points to the monosumoylated DRP1. (C, i and ii) HeLa cells were transfected with YFP–SUMO-1 and treated with STS from 95 to 205 min. YFP–SUMO-1–positive puncta (red; left insets) were detected in association with mitochondria labeled with MitoFluor red (green; middle insets), often at potential fission sites (right insets). The insets show magnifications of the boxed regions. The left inset is a grayscale image of the YFP–SUMO-1 channel (shown in green in the overlay in the right inset), and the middle inset shows a grayscale image of the Mitofluor red channel (indicated in red in the overlay in the right inset). Note the presence of YFP–SUMO-1 at the sites of mitochondrial constriction. (D, i and ii) HeLa cells transfected with YFP–SUMO-1 (red) were treated with STS for 2 h, fixed, and immunostained with antibodies against Tom20 (blue) and DRP1 (green). YFP–SUMO-1 puncta associated with mitochondria are observed to colocalize (solid circles) or not colocalize (dotted circles) with DRP1. Bars (C), 5 μm; (D) 1 μm.
Similar articles
- A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis.
Tanaka A, Youle RJ. Tanaka A, et al. Mol Cell. 2008 Feb 29;29(4):409-10. doi: 10.1016/j.molcel.2008.02.005. Mol Cell. 2008. PMID: 18313377 - 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells.
Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordán J, Schrader M. Gomez-Lazaro M, et al. Free Radic Biol Med. 2008 Jun 1;44(11):1960-9. doi: 10.1016/j.freeradbiomed.2008.03.009. Epub 2008 Mar 20. Free Radic Biol Med. 2008. PMID: 18395527 - Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1.
Santel A, Frank S. Santel A, et al. IUBMB Life. 2008 Jul;60(7):448-55. doi: 10.1002/iub.71. IUBMB Life. 2008. PMID: 18465792 Review. - Mitochondrial outer-membrane permeabilization and remodelling in apoptosis.
Jourdain A, Martinou JC. Jourdain A, et al. Int J Biochem Cell Biol. 2009 Oct;41(10):1884-9. doi: 10.1016/j.biocel.2009.05.001. Epub 2009 May 9. Int J Biochem Cell Biol. 2009. PMID: 19439192 Review.
Cited by
- Mitochondrial protein dysfunction in pathogenesis of neurological diseases.
Wang L, Yang Z, He X, Pu S, Yang C, Wu Q, Zhou Z, Cen X, Zhao H. Wang L, et al. Front Mol Neurosci. 2022 Sep 7;15:974480. doi: 10.3389/fnmol.2022.974480. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157077 Free PMC article. Review. - Dynamin-related protein 1 as a therapeutic target in cardiac arrest.
Sharp WW. Sharp WW. J Mol Med (Berl). 2015 Mar;93(3):243-52. doi: 10.1007/s00109-015-1257-3. Epub 2015 Feb 8. J Mol Med (Berl). 2015. PMID: 25659608 Free PMC article. Review. - Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins.
Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Brooks C, et al. Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11649-54. doi: 10.1073/pnas.0703976104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606912 Free PMC article. - The role of PTEN-induced kinase 1 in mitochondrial dysfunction and dynamics.
Thomas KJ, Cookson MR. Thomas KJ, et al. Int J Biochem Cell Biol. 2009 Oct;41(10):2025-35. doi: 10.1016/j.biocel.2009.02.018. Epub 2009 Mar 5. Int J Biochem Cell Biol. 2009. PMID: 19703660 Free PMC article. Review. - MicroRNAs in cardiac apoptosis.
Li P. Li P. J Cardiovasc Transl Res. 2010 Jun;3(3):219-24. doi: 10.1007/s12265-010-9175-9. Epub 2010 Mar 19. J Cardiovasc Transl Res. 2010. PMID: 20560043 Review.
References
- Antonsson, B., S. Montessuit, B. Sanchez, and J.C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623. - PubMed
- Arnoult, D., N. Rismanchi, A. Grodet, R.G. Roberts, D.P. Seeburg, J. Estaquier, M. Sheng, and C. Blackstone. 2005. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr. Biol. 15:2112–2118. - PubMed
- Basanez, G., A. Nechushtan, O. Drozhinin, A. Chanturiya, E. Choe, S. Tutt, K.A. Wood, Y. Hsu, J. Zimmerberg, and R.J. Youle. 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA. 96:5492–5497. - PMC - PubMed
- Basanez, G., J.C. Sharpe, J. Galanis, T.B. Brandt, J.M. Hardwick, and J. Zimmerberg. 2002. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277:49360–49365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous